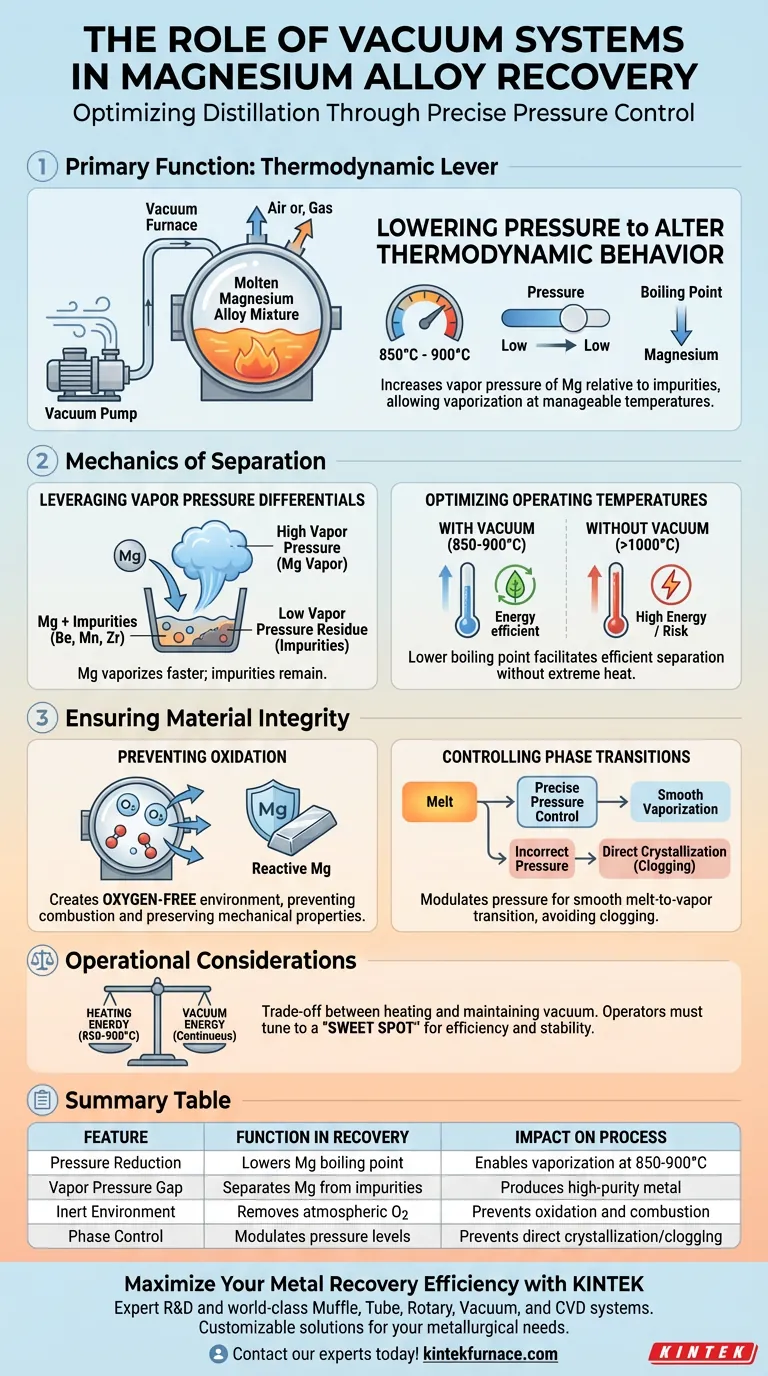
The primary function of the vacuum system in magnesium recovery is to drastically lower the environmental pressure to alter the thermodynamic behavior of the metal. By reducing pressure, the system increases the vapor pressure of magnesium relative to other elements, allowing it to vaporize preferentially at manageable temperatures between 850°C and 900°C. This mechanism separates magnesium from impurities based on volatility while simultaneously creating an oxygen-free environment to prevent combustion or oxidation.
The vacuum system acts as a thermodynamic lever, utilizing pressure differentials to separate magnesium from refractory impurities that would otherwise be difficult to remove, ensuring high purity without damaging the highly reactive metal.

The Mechanics of Separation
Leveraging Vapor Pressure Differentials
The core principle of this process is the significant difference in vapor pressure between magnesium and impurity elements. Under vacuum conditions, magnesium exhibits a much higher vapor pressure than refractory elements such as beryllium, manganese, and zirconium.
This difference implies that magnesium will turn into a gas (vaporize) much faster and more readily than these contaminants. Consequently, the magnesium leaves the mix as a vapor, while the impurities remain behind as a solid or liquid residue in the furnace.
Optimizing Operating Temperatures
The vacuum environment allows the process to occur efficiently at temperatures between 850°C and 900°C. Without a vacuum, achieving the same rate of vaporization would require significantly higher temperatures, which could be energy-inefficient or damaging to the equipment.
By lowering the boiling point, the system facilitates effective metal separation without requiring extreme thermal input.
Ensuring Material Integrity
Preventing Oxidation
Magnesium and its alloys are highly chemically active and prone to rapid oxidation at high temperatures. Even trace amounts of oxygen can degrade the material or pose safety risks.
The vacuum system removes atmospheric gases, including oxygen, from the furnace chamber. This creates an inert environment that protects the magnesium matrix, ensuring the recovered metal retains superior mechanical properties and clean interfaces.
Controlling Phase Transitions
The vacuum level must be carefully modulated to manage how magnesium changes states. The system is designed to prevent issues such as the direct crystallization of magnesium from the melt, which can occur if conditions are not balanced correctly.
By maintaining specific pressure and temperature parameters, the system ensures a smooth transition from melt to vapor, facilitating a continuous and efficient separation process.
Operational Considerations and Trade-offs
Balancing Vacuum Levels
While a vacuum is essential, an ultra-high vacuum is not always the target for distillation efficiency. The primary reference notes that specific vacuum conditions are needed to prevent direct crystallization issues that might arise under ultra-high vacuum settings.
Operators must tune the vacuum to a "sweet spot" that maximizes vaporization rates without inducing unwanted phase changes that could clog the system or trap impurities.
Thermal Management vs. Pressure
There is a trade-off between the energy required to heat the furnace and the energy required to maintain the vacuum.
Operating at 850-900°C requires robust heating elements, but the vacuum reduces the thermal load compared to atmospheric distillation. However, the system requires continuous energy to maintain low pressure against the evolution of magnesium vapor.
Making the Right Choice for Your Goal
To optimize the recovery of magnesium alloys, you must align the vacuum system's capabilities with your specific purity and efficiency targets.
- If your primary focus is High Purity: Prioritize a system capable of stable pressure control to maximize the vapor pressure gap between magnesium and impurities like iron, silicon, and aluminum.
- If your primary focus is Process Stability: Ensure the vacuum system can modulate pressure within the 850-900°C window to avoid direct crystallization and ensure smooth vaporization.
By precisely controlling the vacuum environment, you convert the volatility of magnesium from a liability into its greatest purification asset.
Summary Table:
| Feature | Function in Magnesium Recovery | Impact on Process |
|---|---|---|
| Pressure Reduction | Lowers the boiling point of magnesium | Enables vaporization at 850°C - 900°C |
| Vapor Pressure Gap | Separates Mg from refractory impurities | Produces high-purity recovered metal |
| Inert Environment | Removes atmospheric oxygen | Prevents metal combustion and oxidation |
| Phase Control | Modulates pressure levels | Prevents direct crystallization/clogging |
Maximize Your Metal Recovery Efficiency with KINTEK
Precise vacuum control is the difference between high-purity magnesium and costly oxidation losses. KINTEK provides the cutting-edge thermal technology your laboratory needs to master these complex phase transitions.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique distillation and metallurgical requirements.
Ready to optimize your magnesium recovery process? Contact our experts today to find your custom solution!
Visual Guide
References
- В. Н. Володин, Xeniya Linnik. Recycling of beryllium, manganese, and zirconium from secondary alloys by magnesium distillation in vacuum. DOI: 10.31643/2024/6445.42
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why is graphite the material of choice for vacuum furnaces? Unmatched Performance in Extreme Heat
- What is the purpose of using a high-vacuum system and annealing furnace for Mn–Ni–Fe–Si alloys? Unlock Data Accuracy
- What are the key benefits of using a vacuum sintering furnace? Achieve Superior Material Purity and Process Control
- Why use a vacuum drying oven for mesoporous silica? Protect High Surface Area and Structural Integrity
- How does a high vacuum furnace ensure the purity of Ti-Nb alloys? Expert Guide to Sintering and Debinding
- Why are vacuum annealing furnaces widely used in the metal heat treatment industry? Unlock Precision and Clean Results
- How does a vacuum furnace create its working environment? Uncover the Secrets to Purity and Precision
- What is the core technical mechanism of low-oxygen brazing? Master Oxide Decomposition for Perfect Bonds



















