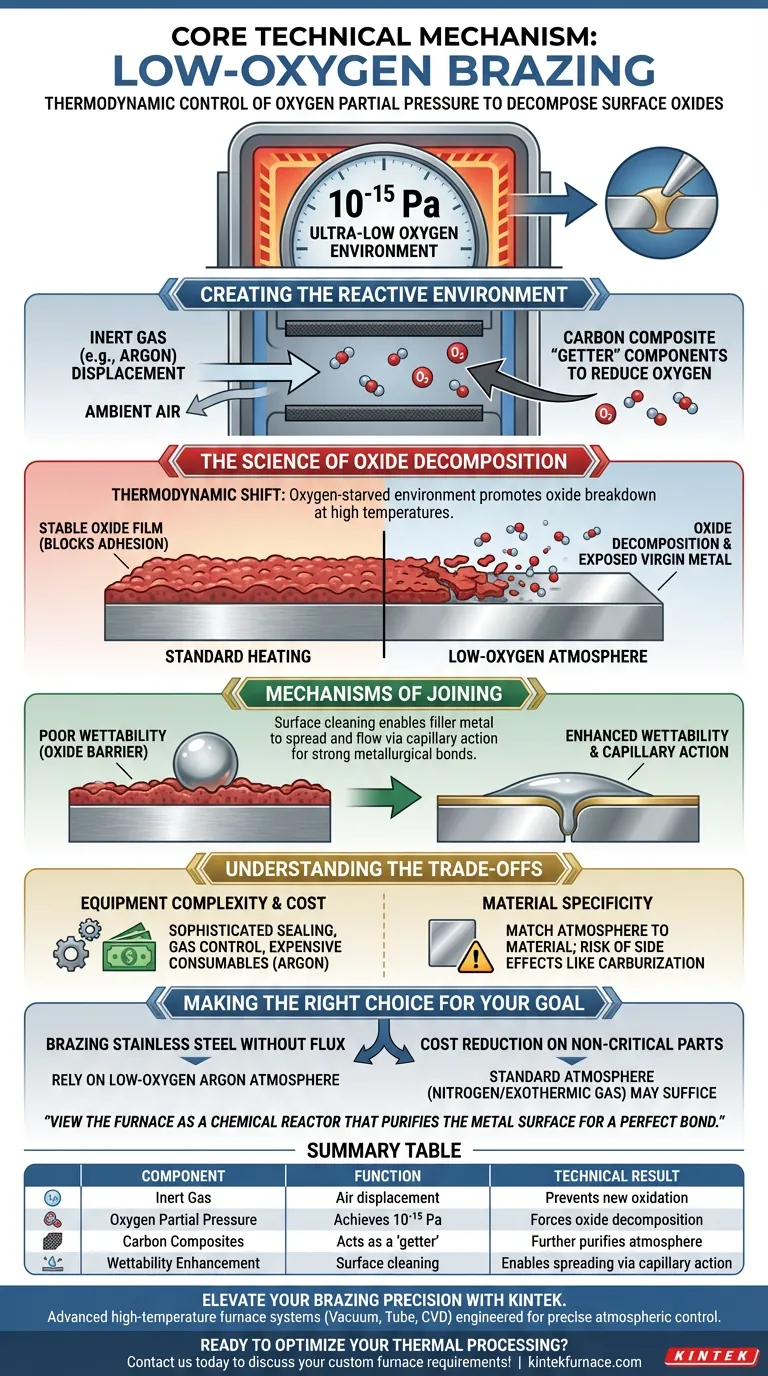
The core technical mechanism is the thermodynamic control of oxygen partial pressure to decompose surface oxides. By utilizing inert gases like argon and carbon composite components, the furnace reduces oxygen levels to approximately 10⁻¹⁵ Pa. This ultra-low oxygen environment not only prevents new oxidation but actively causes existing oxide films on metals like stainless steel to decompose, creating a pristine surface for the filler metal to wet and spread.
Success in low-oxygen brazing relies on manipulating the atmosphere to favor pure metal over metal oxides. By achieving an ultra-low oxygen partial pressure, the process removes chemical barriers, allowing the filler metal to form strong metallurgical bonds through enhanced wettability.

Creating the Reactive Environment
The Role of Inert Gases
The foundation of this process involves displacing atmospheric air with protective gases. Inert gases, such as argon, are introduced into the furnace chamber to create a barrier against ambient oxygen.
Achieving Ultra-Low Partial Pressure
Displacement alone is often insufficient for high-quality brazing; the system must achieve an extremely low oxygen partial pressure, specifically around 10⁻¹⁵ Pa. This level of purity is often assisted by carbon composite conveyor belts, which can act as a "getter" to further reduce oxygen presence within the heated zone.
The Science of Oxide Decomposition
Reversing Oxidation
Standard heating causes metals to form stable oxide films that block adhesion. However, in this specific low-oxygen environment, the thermodynamics shift. The atmosphere is so oxygen-starved that it promotes the decomposition of existing surface oxides at high temperatures.
Exposing the Substrate
As the oxide film breaks down, the underlying "virgin" metal is exposed. This is critical for materials like stainless steel, where the passive oxide layer is notoriously difficult to penetrate without aggressive chemical fluxes.
Mechanisms of Joining
Enhancing Wettability
For a braze to work, the molten filler must be able to "wet" the base metal. Oxide films prevent this, causing the filler to bead up. By decomposing the oxides, the furnace significantly enhances the spreading capability of the brazing filler metal across the component surface.
Facilitating Capillary Action
Once wettability is achieved, physical mechanics take over. Capillary action draws the liquid filler metal into the tight clearance between the parts. Without the oxide barrier, the filler flows smoothly and solidifies to form a consistent metallurgical bond.
Understanding the Trade-offs
Equipment Complexity and Cost
Achieving a partial pressure of 10⁻¹⁵ Pa requires sophisticated sealing, gas control systems, and expensive consumables like argon. This is a significantly more complex and costly process than standard air brazing or lower-grade atmosphere brazing.
Material Specificity
While ideal for stainless steel, the atmosphere must be carefully matched to the material. Mismanagement of the gas mixture (e.g., introducing reactive gases like hydrogen or nitrogen when not appropriate) can lead to unintended side effects like carburization or embrittlement, rather than simple oxide reduction.
Making the Right Choice for Your Goal
To determine if this process aligns with your manufacturing requirements, consider the following:
- If your primary focus is brazing stainless steel without flux: Rely on the low-oxygen argon atmosphere to decompose the passive oxide layer naturally.
- If your primary focus is cost reduction on non-critical parts: A standard atmosphere furnace using nitrogen or simple exothermic gas may be sufficient, provided the joint tolerance for oxidation is higher.
Mastering low-oxygen brazing requires viewing the furnace not just as a heater, but as a chemical reactor that purifies the metal surface for a perfect bond.
Summary Table:
| Mechanism Component | Function | Technical Result |
|---|---|---|
| Inert Gas (Argon) | Air displacement | Prevents new oxidation during heating |
| Oxygen Partial Pressure | Achieves 10⁻¹⁵ Pa | Forces existing surface oxides to decompose |
| Carbon Composites | Acts as a "getter" | Further purifies the local furnace atmosphere |
| Wettability Enhancement | Surface cleaning | Enables filler metal to spread via capillary action |
Elevate Your Brazing Precision with KINTEK
Don’t let surface oxides compromise your joint integrity. KINTEK’s advanced high-temperature furnace systems—including Vacuum, Tube, and CVD furnaces—are engineered to provide the precise atmospheric control required for ultra-low oxygen brazing.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet the unique material demands of your laboratory or production line. Whether you are brazing stainless steel or developing specialized alloys, we provide the technical edge you need to ensure flawless metallurgical bonds.
Ready to optimize your thermal processing? Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Yoshio Bizen, Yasuyuki Miyazawa. Brazing of Ferritic Stainless Steel with Ni-25Cr-6P-1.5Si-0.5B-1.5Mo Amorphous Brazing Foil Having a Liquidus of 1243 K with Continuous Conveyor Belt Furnace in Low-Oxygen Atmosphere. DOI: 10.2320/matertrans.mt-m2023207
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What role does a high-temperature furnace play in CsPbCl3-MSN synthesis? Mastering Thermal Encapsulation
- What are the main components of a vacuum annealing furnace? Essential Systems for Precision Heat Treatment
- How is chamber customization beneficial in vacuum furnaces? Boost Purity, Efficiency, and Performance
- How is cooling achieved in a vacuum furnace after the desired process? Master Gas Quenching for Precision Results
- Why are multiple heat treatment cycles in a pyrolysis furnace necessary for dense SiC matrix formation in PIP?
- What are the advantages of using a vacuum annealing furnace? Achieve Clean, Oxidation-Free Heat Treatment
- Why is Spark Plasma Sintering (SPS) optimal for Ti2AlN ceramics? Achieving 99.2% Purity and Maximum Density
- What are the types of vacuum furnaces based on heating form? Internal vs. External Heating Explained



















