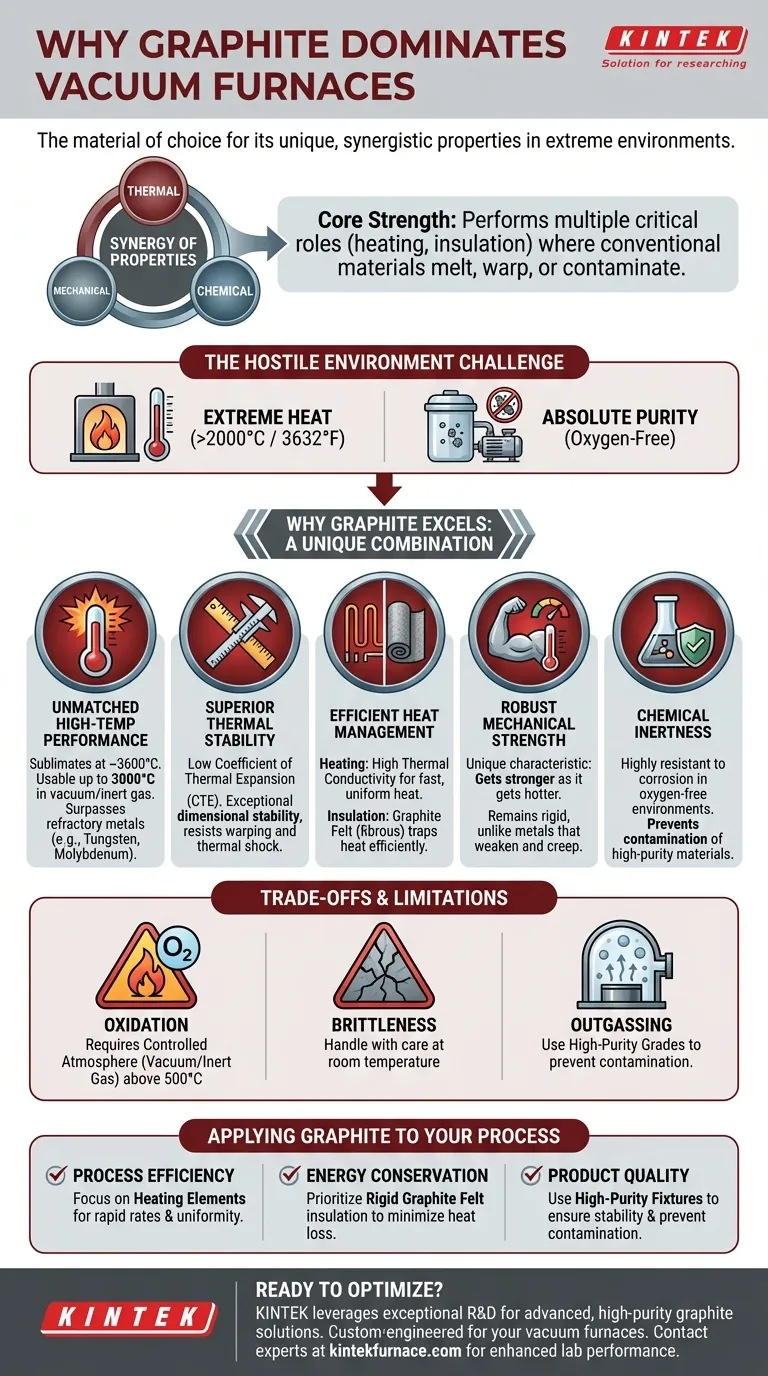
At its core, graphite is the material of choice for vacuum furnaces because it possesses a unique combination of properties that no other practical material can match. It withstands extreme temperatures far beyond the melting point of most metals, maintains its structural integrity under intense thermal stress, and remains chemically stable in the high-vacuum, inert environments where these processes occur.
The decision to use graphite is not based on a single characteristic, but on the synergy of its thermal, mechanical, and chemical properties. This synergy allows it to perform multiple critical roles—from generating heat to providing insulation—in an environment that would cause conventional materials to melt, warp, or contaminate the process.

The Core Challenge: The Hostile Environment of a Vacuum Furnace
A vacuum furnace is designed for heat-treating materials in a controlled atmosphere, free from oxygen and other contaminants. This environment is defined by two primary challenges: extreme heat and the need for absolute purity.
The materials used to build the furnace's "hot zone"—the area where heating occurs—must be able to function reliably at temperatures that can exceed 2000°C (3632°F) without degrading, warping, or reacting with the products being treated.
Why Graphite Excels: A Unique Combination of Properties
Graphite's suitability comes from its ability to solve all of the furnace's core challenges simultaneously.
Unmatched High-Temperature Performance
Unlike metals that melt, graphite sublimates (turns directly from a solid to a gas) at an extremely high temperature, around 3600°C. In the inert gas or vacuum conditions of a furnace, it can be used reliably up to 3000°C, far surpassing the operational limits of refractory metals like molybdenum or tungsten in many applications.
Superior Thermal Stability
Graphite has a very low coefficient of thermal expansion (CTE). This means it barely expands or contracts when heated or cooled, giving it incredible dimensional stability and resistance to warping.
This property also gives it powerful resistance to thermal shock. It can withstand rapid temperature changes that would cause ceramics to crack or metals to deform.
Efficient Heat Management
Graphite serves a dual role in heat management. In its solid form, it has high thermal conductivity, making it an excellent material for heating elements that deliver fast, uniform temperature control.
Conversely, when processed into a fibrous mat known as graphite felt, it becomes an exceptional insulator. This lightweight felt is used to line the hot zone, trapping heat with remarkable efficiency.
Robust Mechanical Strength
A unique characteristic of graphite is that it gets stronger as it gets hotter. While metals weaken and creep at high temperatures, graphite's tensile strength increases, ensuring fixtures and components remain rigid.
It is also lightweight and easily machinable, which reduces the structural load on the furnace and allows for the creation of complex fixtures and tooling at a lower cost.
Chemical Inertness
Graphite is highly resistant to chemical attack and corrosion. Within the oxygen-free environment of a vacuum furnace, it is exceptionally non-reactive, which prevents it from contaminating the high-purity materials being processed.
Understanding the Trade-offs and Limitations
While graphite is the dominant material, it is not without specific operational requirements. Its primary limitation is its susceptibility to oxidation.
The Need for a Controlled Atmosphere
Graphite will begin to rapidly burn away (oxidize) in the presence of oxygen at temperatures above approximately 500°C. This is precisely why it is exclusively used in vacuum or inert gas furnaces, where oxygen is removed to prevent this reaction.
Handling and Brittleness
At room temperature, graphite can be brittle compared to metals. Components must be handled with care during installation and maintenance to prevent chipping or cracking.
Potential for Outgassing
If not properly purified and prepared, graphite can contain trapped gases and moisture. When heated in a vacuum, this can lead to outgassing, which can contaminate the furnace environment and the workpiece. High-purity grades are used to mitigate this risk.
How to Apply This to Your Process
Your choice of graphite components should directly align with your primary operational goal.
- If your primary focus is process efficiency: Invest in high-quality, well-designed graphite heating elements to ensure rapid heating rates and excellent temperature uniformity.
- If your primary focus is energy conservation: Prioritize rigid graphite felt insulation with low thermal conductivity to minimize heat loss and reduce power consumption.
- If your primary focus is product quality: Use high-purity, precisely machined graphite fixtures and tooling to guarantee part stability and prevent contamination during the heat-treating cycle.
Ultimately, understanding graphite's properties empowers you to optimize every aspect of your high-temperature vacuum process.
Summary Table:
| Key Property | Why It Matters for Vacuum Furnaces |
|---|---|
| Extreme Temp. Resistance | Sublimates at ~3600°C; usable up to 3000°C in vacuum/inert gas. |
| Low Thermal Expansion | Exceptional dimensional stability; resists warping from thermal stress. |
| High Thermal Conductivity | Ideal for efficient, uniform heating elements. |
| High-Temperature Strength | Gets stronger when heated, unlike metals that weaken. |
| Chemical Inertness | Non-reactive in oxygen-free environments, preventing contamination. |
Ready to optimize your high-temperature processes with superior graphite solutions?
KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced, high-purity graphite components for vacuum furnaces. Our deep customization capabilities ensure your heating elements, insulation, and fixtures are precisely engineered for maximum efficiency, energy conservation, and product quality.
Contact our experts today to discuss how our graphite expertise can enhance your lab's performance.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What is the primary function of a vacuum graphite furnace? Achieve Extreme-Temperature Material Purity
- What is the mechanism and effect of post-annealing NiTi thin films in a vacuum furnace? Unlock Superelasticity
- How does graphite contribute to energy efficiency in vacuum furnaces? Achieve Faster, More Uniform Heating
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- Why are vacuum furnaces used for the re-quenching of samples after a boriding treatment? Master Core Toughness



















