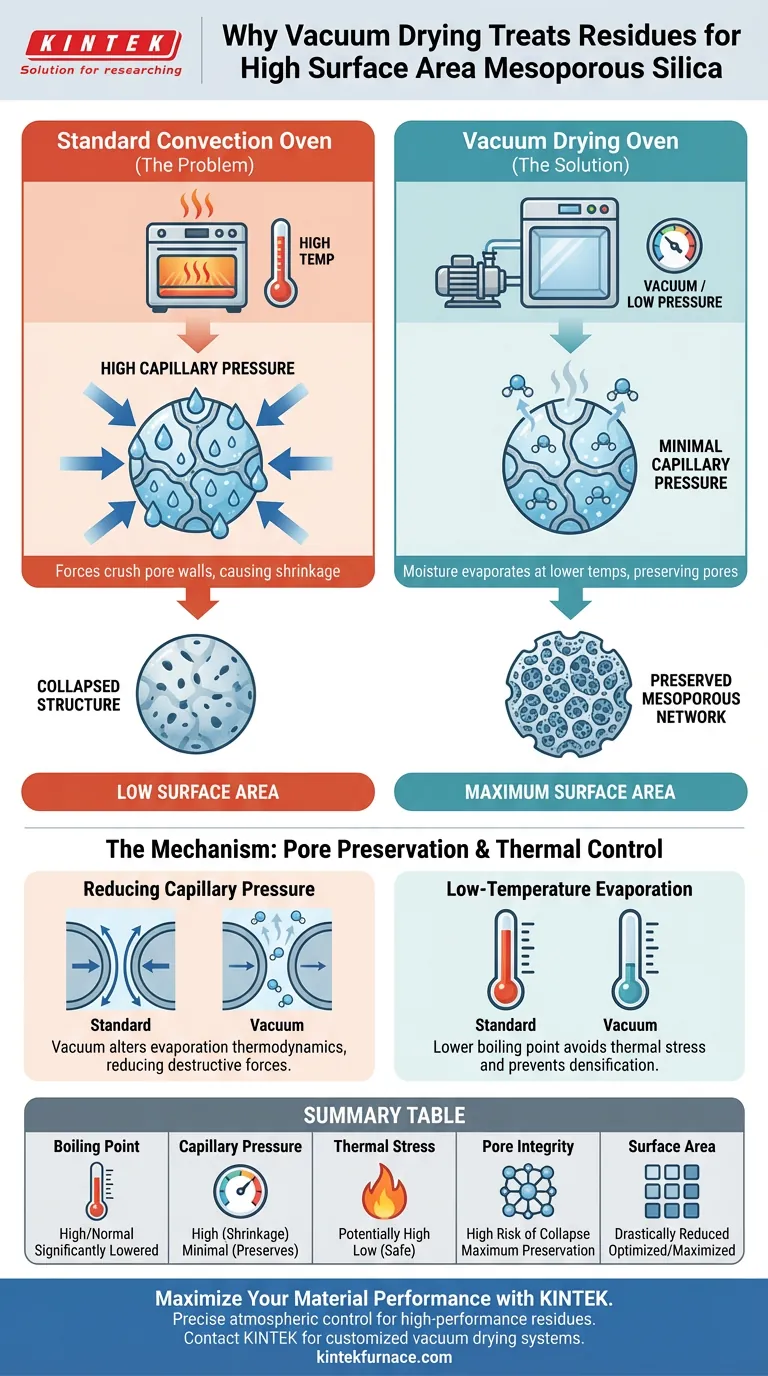
The primary function of a vacuum drying oven in this context is to minimize capillary pressure during moisture removal. When preparing mesoporous silica, specifically after acid etching, the internal structure is delicate and filled with fluid. Vacuum drying allows this moisture to evaporate at significantly lower temperatures, preventing the high surface tension forces of evaporating liquid from crushing the pore walls and destroying the high surface area.
Core Takeaway The transition from a wet residue to a dry solid is the most critical moment for porous materials. By operating in a vacuum, you lower the boiling point of the solvent, eliminating the strong capillary forces that cause structural shrinkage. This preserves the expansive mesoporous network essential for the material's performance.

The Mechanism of Pore Preservation
Reducing Capillary Pressure
The central challenge in drying porous materials is the physical force exerted by the liquid as it leaves the pore. As water or solvent evaporates under standard atmospheric conditions, it creates significant capillary pressure against the pore walls.
In a mesoporous structure, these forces are strong enough to pull the walls inward. Using a vacuum environment alters the thermodynamics of evaporation, effectively reducing this pressure and allowing the moisture to exit without exerting destructive force on the silica framework.
Preventing Structural Collapse
During the preparation of mesoporous silica, the structure is often formed through processes like acid etching. This creates a rich, but fragile, network of voids.
If this residue is dried in a standard convection oven, the capillary forces mentioned above lead to shrinkage or total collapse of the pores. Vacuum drying prevents this collapse, ensuring the material retains the specific morphology intended during the synthesis phase.
Thermal Control and Surface Area
Low-Temperature Evaporation
A vacuum drying oven reduces the internal pressure of the chamber, which directly lowers the boiling point of the residual moisture or solvents.
This allows the residue to be dried thoroughly at lower temperatures. By avoiding high thermal stress, you protect the material from densification that often occurs when silica is subjected to high heat while still wet.
Maximizing Specific Surface Area
The ultimate metric for the quality of mesoporous silica is its specific surface area. This is the direct result of keeping the pores open.
By utilizing vacuum drying to mitigate shrinkage, the final product retains the maximum possible surface area. This renders the silica highly effective for downstream applications, such as adsorption or catalysis, where surface interaction is key.
Understanding the Trade-offs
Process Complexity vs. Material Quality
While vacuum drying is superior for quality, it introduces operational complexity compared to standard drying. It is typically a batch process that requires maintaining a consistent seal and monitoring pressure levels.
However, for mesoporous materials, this is a necessary trade-off. Attempting to speed up the process using standard high-heat methods will almost invariably result in a material with a drastically reduced surface area, rendering the synthesis effort wasted.
Making the Right Choice for Your Goal
To determine if vacuum drying is strictly necessary for your specific silica application, evaluate your performance metrics:
- If your primary focus is Maximum Surface Area: You must use vacuum drying to eliminate capillary forces and prevent pore collapse during the solvent removal phase.
- If your primary focus is Structural Integrity: You should rely on the low-temperature capabilities of the vacuum oven to avoid thermal shrinkage of the delicate etched framework.
Vacuum drying is not merely a method of heating; it is a structural preservation technique essential for high-performance porous materials.
Summary Table:
| Feature | Standard Convection Oven | Vacuum Drying Oven |
|---|---|---|
| Boiling Point | Normal (Higher) | Significantly Lowered |
| Capillary Pressure | High (Causes Shrinkage) | Minimal (Preserves Pores) |
| Thermal Stress | Potentially High | Low (Safe for Residues) |
| Pore Integrity | High Risk of Collapse | Maximum Preservation |
| Surface Area | Drastically Reduced | Optimized / Maximized |
Maximize Your Material Performance with KINTEK
Preserving the delicate architecture of mesoporous silica requires more than just heat—it requires precise atmospheric control. KINTEK provides industry-leading vacuum drying systems designed to eliminate capillary pressure and protect your high-performance residues.
Backed by expert R&D and manufacturing, KINTEK offers a full suite of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory or industrial needs. Whether you are scaling up catalyst production or refining material synthesis, our technology ensures your structural integrity remains uncompromised.
Ready to optimize your drying process? Contact KINTEK today for a customized solution.
Visual Guide
References
- Jian-ming Gao, Yanxia Guo. Novel process for high value utilization of high-alumina fly ash: valuable metals recovery and mesoporous silica <i>in situ</i> preparation. DOI: 10.1039/d3ra06921d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is an industrial-grade high-temperature sintering furnace required for the production of multi-channel ceramic membrane supports? Key Roles in Material Transformation
- What role do vacuum annealing furnaces play in optical material processing? Enhance Clarity and Performance for Your Optics
- Why is vacuum annealing important for rare metal materials? Ensure Purity and Performance in Critical Applications
- What role do laboratory arc furnaces and tungsten electrodes play in TiCo1-xCrxSb synthesis? Expert Material Analysis
- How does a bell-type plasma nitriding furnace enhance GGG60 ductile iron? Superior Surface Hardening Solutions
- What advantages does a vacuum drying oven offer over a standard oven for Fe3Al and CNTs? Protect Your Composites
- What are the main structural components of a vacuum sintering furnace? Unlock Precision in High-Temperature Processing
- What is the purpose of transferring high-temperature glass to a preheated annealing furnace? Ensuring Sample Integrity



















