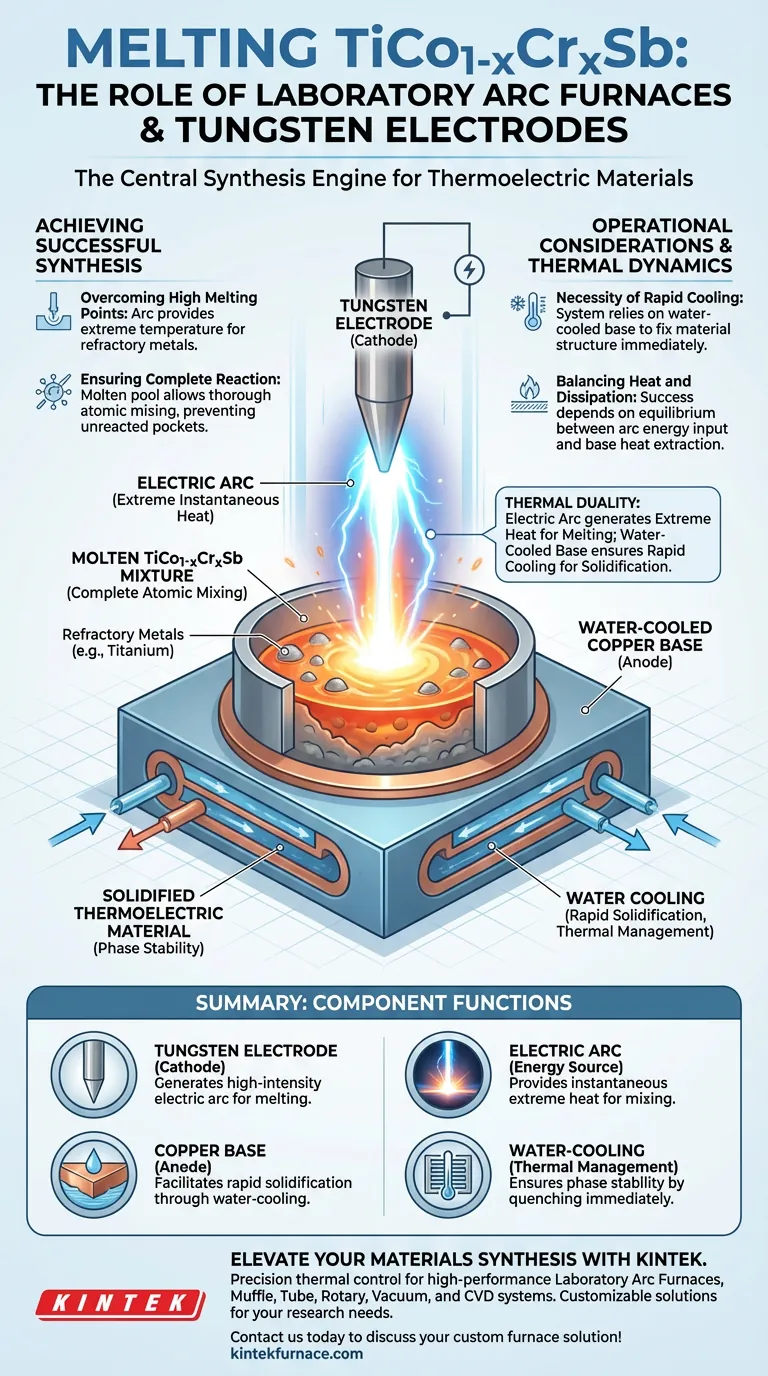
Laboratory arc furnaces serve as the central synthesis engine for TiCo1-xCrxSb thermoelectric materials, providing the extreme thermal environment necessary to fuse raw components. A tungsten electrode acts as the cathode, generating an intense electric arc against a water-cooled copper anode to instantaneously melt high-melting-point metals while facilitating rapid solidification.
The synthesis process relies on a critical thermal duality: the electric arc generates the extreme heat needed to fully react refractory metals, while the water-cooled base ensures the rapid cooling essential for proper material solidification.
The Mechanics of Arc Melting
The Role of the Tungsten Electrode
In this specific setup, the tungsten electrode functions as the cathode. It is the source of the electric arc, channeling high-energy current to the raw materials.
Because tungsten has an incredibly high melting point itself, it can sustain the arc without degrading, delivering the energy required to melt the target materials.
The Function of the Water-Cooled Anode
Opposite the electrode sits a copper base that acts as the anode. Crucially, this base is water-cooled.
This cooling mechanism is not merely for safety; it is an active participant in the synthesis, designed to absorb heat rapidly once the reaction is complete.
Achieving Successful Synthesis
Overcoming High Melting Points
The synthesis of TiCo1-xCrxSb involves metals with high melting points, such as titanium. The laboratory arc furnace provides extremely high instantaneous temperatures that standard furnaces may struggle to achieve.
This intensity ensures that even the most refractory components in the mixture are fully melted.
Ensuring Complete Reaction
For the thermoelectric material to function correctly, the raw components must undergo a full reaction.
The electric arc facilitates this by creating a molten pool where the elements can mix thoroughly at the atomic level, preventing unreacted pockets of raw metal.
Operational Considerations and Thermal Dynamics
The Necessity of Rapid Cooling
This method introduces a specific operational constraint: the management of extreme heat. The process does not allow for gradual cooling.
The system relies heavily on the water-cooled base to achieve rapid solidification. This sudden drop in temperature is required to fix the material's structure immediately after the components have fused.
Balancing Heat and Dissipation
The success of the process depends on the balance between the arc's energy input and the base's heat extraction.
The high instantaneous temperature ensures reaction, but without the active cooling of the copper base, the material could not solidify at the rate necessary for the desired material properties.
Making the Right Choice for Your Goal
To optimize the synthesis of TiCo1-xCrxSb, focus on the specific functions of the furnace components:
- If your primary focus is compositional homogeneity: Ensure the tungsten electrode generates sufficient arc intensity to fully melt the highest melting-point component in your matrix.
- If your primary focus is phase stability: Rely on the efficiency of the water-cooled copper base to drive the rapid cooling rates required for immediate solidification.
By mastering the interplay between the tungsten cathode's heat and the copper anode's cooling, you ensure the creation of high-quality thermoelectric materials.
Summary Table:
| Component | Role in Process | Primary Function |
|---|---|---|
| Tungsten Electrode | Cathode | Generates high-intensity electric arc for melting high-melting-point metals |
| Copper Base | Anode | Facilitates rapid solidification through integrated water-cooling |
| Electric Arc | Energy Source | Provides instantaneous extreme heat for complete atomic mixing |
| Water-Cooling | Thermal Management | Ensures phase stability by quenching the material immediately after fusion |
Elevate Your Materials Synthesis with KINTEK
Precision in thermoelectric material production requires specialized thermal control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Laboratory Arc Furnaces, Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable for your unique laboratory needs.
Whether you are synthesizing refractory alloys or developing next-generation thermoelectrics, our advanced heating solutions provide the reliability and temperature precision your research demands.
Ready to optimize your lab's capabilities? Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Volodymyr Krayovskyy, А. Horyn. SIMULATION OF CHARACTERISTICS OF SENSITIVE ELEMENTS OF TEMPERATURE CONVERTERS BASED ON TiCo1-xCrxSb. DOI: 10.23939/istcmtm2024.04.030
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why is a vacuum preheating furnace used for aluminum foam sandwich panels? Ensure High-Strength Metallic Bonding
- In which industries is the vacuum carburizing furnace commonly used? Essential for Aerospace and High-Performance Machinery
- How does the combined use of a centrifuge and a vacuum drying oven solve issues in H-Beta zeolite catalyst recovery?
- Why is vacuum brazing considered cost-effective? Reduce Costs with High-Quality Joints
- What is the function of a vacuum system in the vacuum distillation recovery process for magnesium alloys?
- What are the typical vacuum levels for medium, high, and ultra-high vacuum furnaces? Optimize Your Process Efficiency
- Why is vacuum brazing considered a clean process? Achieve Oxide-Free, Flux-Free Metal Joining
- What automation features are present in modern vacuum furnaces? Boost Precision and Efficiency in Your Lab



















