Temperature control in the condenser is the critical variable that determines both the physical state and the chemical purity of the recovered magnesium. By strictly regulating thermal conditions, operators manage the conversion of gaseous magnesium into specific solid or liquid forms, directly influencing the efficiency of the entire distillation process.
Core Takeaway: Precise thermal regulation in the condenser is not just about state conversion; it is the primary mechanism for separating pure magnesium from refractory contaminants, thereby defining the quality of both the recovered metal and the byproduct master alloys.

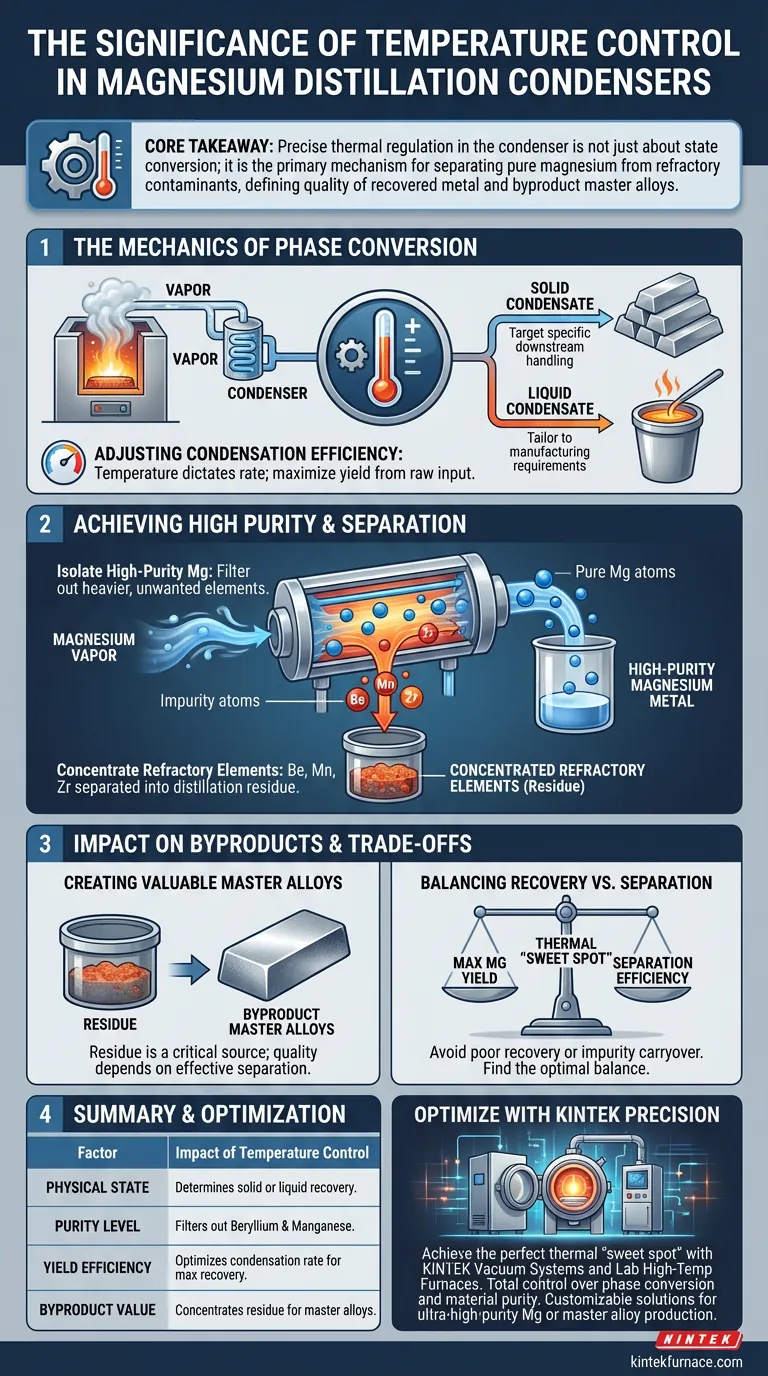
The Mechanics of Phase Conversion
Determining the Condensate Form
The fundamental role of the condenser is to transition magnesium from a gaseous state back into a usable physical form.
Depending on the specific temperature settings applied, the system can target the production of either solid or liquid condensates. This flexibility allows the process to be tailored to specific downstream handling or manufacturing requirements.
Adjusting Condensation Efficiency
Temperature directly dictates the efficiency of the condensation process.
By manipulating the thermal gradient, operators can maximize the rate at which magnesium is captured from the vapor stream. This ensures the highest possible yield of the metal from the raw input.
Achieving High Purity and Separation
Isolating High-Purity Magnesium
The distillation process relies on temperature control to act as a filter.
When the condenser is operated at optimal temperatures, it facilitates the recovery of high-purity magnesium metal. This ensures that the final magnesium product is free from heavier, unwanted elements that do not vaporize or condense under the same conditions.
Concentrating Refractory Elements
A key function of the condenser temperature is to ensure that specific refractory elements do not contaminate the magnesium.
Elements such as beryllium, manganese, and zirconium are effectively separated during this stage. Because they are not collected in the condenser with the magnesium, they are concentrated in the distillation residue.
Impact on Byproducts
Creating Valuable Master Alloys
The residue left behind is not merely waste; it is a critical source for secondary products.
By ensuring refractory elements remain in the residue, the process creates a concentrated base for byproduct master alloys. The quality and chemical composition of these alloys are directly dependent on how effectively the magnesium was separated in the condenser.
Understanding the Operational Trade-offs
Balancing Recovery vs. Separation
There is often a delicate balance between maximizing magnesium yield and maintaining separation efficiency.
If the temperature profile is incorrect, you risk either poor magnesium recovery rates or the carryover of impurities. The goal is to find the thermal "sweet spot" that recovers the maximum amount of magnesium while keeping refractory elements strictly confined to the residue.
Making the Right Choice for Your Goal
To optimize the magnesium distillation process, you must adjust your thermal parameters based on your desired end-product.
- If your primary focus is High-Purity Magnesium: Prioritize condenser temperatures that maximize condensation efficiency to capture the magnesium vapor while excluding heavier elements.
- If your primary focus is Master Alloy Production: Ensure the separation process is rigorous enough to fully concentrate beryllium, manganese, and zirconium in the distillation residue.
Ultimately, the condenser temperature is the lever that controls the distribution of value between your purified metal and your alloy feedstock.
Summary Table:
| Factor | Impact of Temperature Control |
|---|---|
| Physical State | Determines if magnesium recovers as a solid or liquid. |
| Purity Level | Filters out refractory elements like Beryllium and Manganese. |
| Yield Efficiency | Optimizes the condensation rate to maximize metal recovery. |
| Byproduct Value | Concentrates refractory elements in residue for master alloys. |
Optimize Your Metal Distillation with KINTEK Precision
Achieving the perfect thermal 'sweet spot' in magnesium distillation requires equipment that delivers uncompromising accuracy. KINTEK provides industry-leading Vacuum systems and Lab High-Temp Furnaces designed to give you total control over phase conversion and material purity.
Backed by expert R&D and manufacturing, our systems are fully customizable to meet the unique challenges of your refining process. Whether you are targeting ultra-high-purity magnesium or concentrated master alloy production, KINTEK has the solution.
Ready to elevate your lab's performance? Contact us today to discuss your custom high-temperature furnace needs with our engineering team!
Visual Guide
References
- В. Н. Володин, Xeniya Linnik. Recycling of beryllium, manganese, and zirconium from secondary alloys by magnesium distillation in vacuum. DOI: 10.31643/2024/6445.42
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What materials are used for the heating elements in a vacuum furnace? Choose the Right Element for Your High-Temp Needs
- What technological features enhance the efficiency of vacuum furnaces? Boost Performance with Advanced Control & Energy Savings
- How does vacuum sintering improve dimensional tolerances? Achieve Uniform Shrinkage and Precision
- Which materials are suitable for treatment in a vacuum annealing furnace? Protect Reactive Metals and Alloys
- Why must silicon nitride mixed slurries undergo solvent removal in a high-vacuum oven? Ensure Peak Ceramic Integrity
- What pressure range is typically used in a vacuum brazing furnace? Optimize for Clean, Strong Metal Joints
- What is the function of a Vacuum Arc Furnace in the synthesis of MNiSn alloys? Ensure High Purity & Precise Doping
- What are the stages of the heat treatment process in drop-bottom quench furnaces? Achieve Superior Hardness and Strength



















