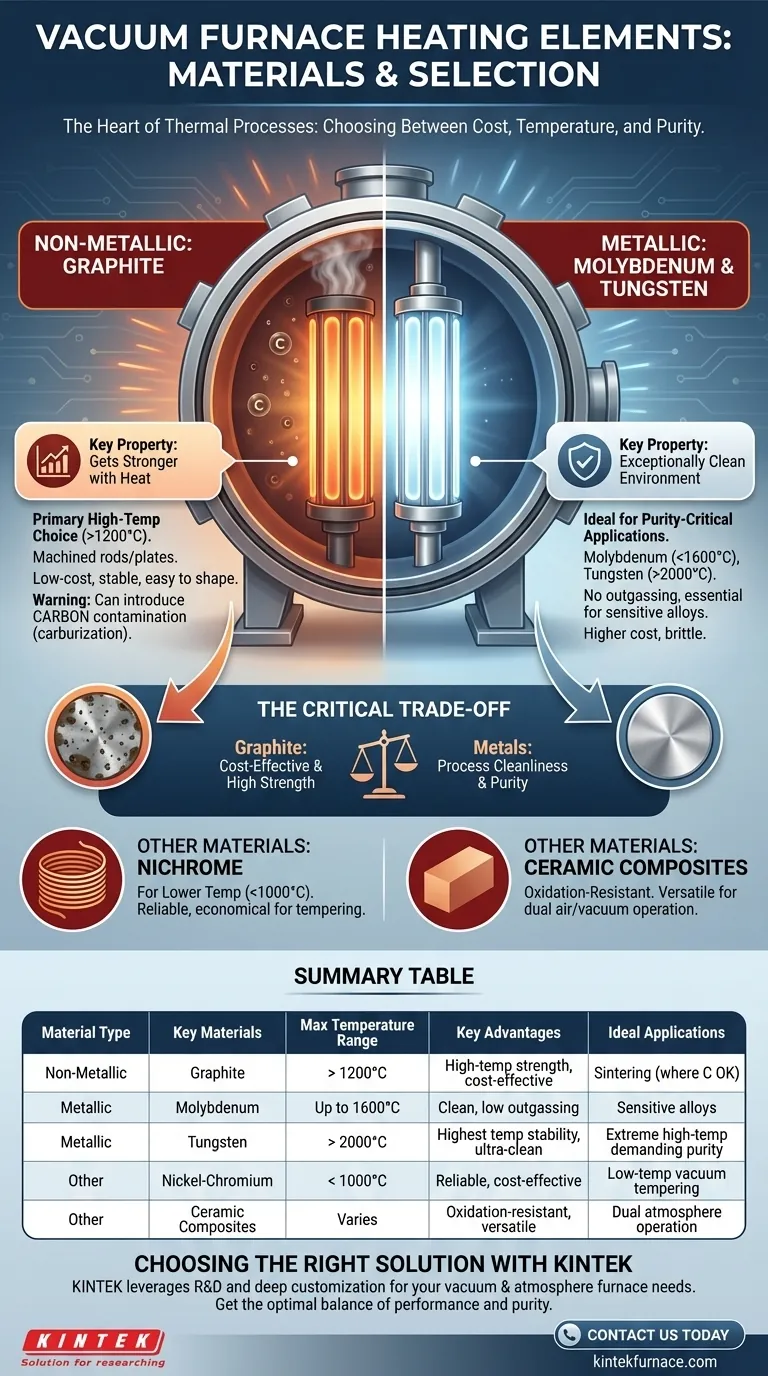
The materials used for heating elements in a vacuum furnace are primarily high-purity graphite or refractory metals like molybdenum and tungsten. The selection is not arbitrary; it is dictated by the furnace's maximum operating temperature, the required vacuum level, and the chemical compatibility with the material being processed. These materials are chosen for their ability to withstand extreme heat, maintain structural integrity, and exhibit very low vapor pressure to avoid contaminating the vacuum environment.
The core decision in vacuum furnace design is a trade-off: graphite offers superior high-temperature performance at a lower cost, while refractory metals provide a much cleaner processing environment, which is critical for sensitive alloys and applications.

The Two Primary Material Families
Heating elements in a vacuum furnace are the heart of the system, responsible for generating the thermal energy for processes like brazing, sintering, and heat-treating. They operate on the principle of resistive heating and are broadly divided into two categories: non-metallic (graphite) and metallic.
Non-Metallic Elements: Graphite
Graphite is the most common heating element material for high-temperature vacuum furnaces, especially for processes operating above 1200°C (2200°F).
It is a form of pure carbon that is machined into rods or plates. Its key advantage is a unique physical property: unlike metals, graphite gets stronger as its temperature increases, making it exceptionally stable at extreme heat. It is also relatively low-cost and easy to machine into complex shapes.
Refractory Metal Elements: Molybdenum and Tungsten
Refractory metals are defined by their incredibly high melting points and resistance to heat. They are the material of choice for applications where process purity is paramount.
Molybdenum (Moly) is the most widely used metallic heating element. It provides a very clean heating environment and is suitable for most vacuum processes up to about 1600°C (2900°F).
Tungsten is used for the most demanding applications requiring temperatures even higher than molybdenum can handle, often exceeding 2000°C (3600°F). It is more expensive and more brittle than molybdenum but offers the ultimate performance in temperature and stability.
Other Specialized Materials
While graphite and refractory metals dominate, other materials are used in specific contexts.
Nickel-Chromium (Nichrome) alloys are often used in lower-temperature vacuum applications, such as tempering, typically below 1000°C (1830°F). They are highly reliable and cost-effective for these less demanding temperature ranges.
Ceramic Composites like molybdenum disilicide (MoSi₂) and silicon carbide (SiC) offer a unique blend of properties, most notably their excellent resistance to oxidation. This makes them suitable for furnaces that may sometimes be operated in air as well as under vacuum.
Understanding the Trade-offs: Graphite vs. Metal
Choosing between a graphite-based "hot zone" and a metallic one is the most significant decision in specifying a vacuum furnace, as it dictates the furnace's capabilities and limitations.
The Contamination Factor
This is the most critical trade-off. Graphite elements can introduce carbon into the furnace atmosphere through outgassing or microscopic particles. This can lead to the carburization of the workpiece surface, a phenomenon that is highly undesirable for many aerospace and medical-grade alloys.
Metallic elements (molybdenum and tungsten) are exceptionally clean. They do not outgas carbon, making them essential for processes requiring the highest purity and for materials that are sensitive to carbon contamination.
Temperature and Atmosphere
Graphite excels at the highest temperatures but is highly reactive with oxygen. A graphite hot zone must never be exposed to air while hot, as the elements will rapidly oxidize and fail.
Refractory metals are also sensitive to oxygen at high temperatures but are perfectly suited for high-vacuum or pure, inert gas (like argon or nitrogen) environments.
Cost and Durability
Graphite elements are generally less expensive to manufacture and replace than their refractory metal counterparts. However, they are more brittle and susceptible to damage from mechanical shock.
Metallic elements are more expensive but can have a long service life if operated correctly. They can, however, become brittle after repeated high-temperature cycles (a process called recrystallization), requiring careful handling during maintenance.
Making the Right Choice for Your Process
The ideal heating element material is directly tied to the desired outcome of your thermal process.
- If your primary focus is maximum temperature and cost-effectiveness (>1200°C): Graphite is the default choice, provided that potential carbon contamination is not a concern for your parts.
- If your primary focus is process cleanliness and purity: Refractory metals like molybdenum or tungsten are non-negotiable to prevent carburization and ensure a pristine vacuum environment.
- If your primary focus is lower-temperature processing (<1000°C): Nickel-chromium alloys provide a reliable and economical solution without the expense of a full refractory metal system.
- If your primary focus is versatile operation in both air and vacuum: Ceramic composite elements like molybdenum disilicide are specifically designed for this operational flexibility.
Understanding these material properties empowers you to select the right furnace technology for your specific engineering goal.
Summary Table:
| Material Type | Key Materials | Max Temperature Range | Key Advantages | Ideal Applications |
|---|---|---|---|---|
| Non-Metallic | Graphite | > 1200°C | High-temperature strength, cost-effective, easy to machine | High-temp processes like sintering, where carbon contamination is acceptable |
| Metallic | Molybdenum | Up to 1600°C | Clean environment, low carbon outgassing | Sensitive alloys in aerospace, medical applications |
| Metallic | Tungsten | > 2000°C | Highest temperature stability, ultra-clean | Extreme high-temp processes demanding purity |
| Other | Nickel-Chromium | < 1000°C | Reliable, cost-effective | Low-temp vacuum applications like tempering |
| Other | Ceramic Composites | Varies | Oxidation-resistant, versatile for air/vacuum | Furnaces requiring dual atmosphere operation |
Struggling to select the right heating element for your vacuum furnace? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements. Whether you need cost-effective graphite elements for high temperatures or ultra-clean refractory metals for sensitive processes, we can help you achieve optimal performance and purity. Contact us today to discuss your application and get a customized solution!
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the function of a vacuum sintering furnace in CoNiCrAlY coatings? Repairing Cold-Sprayed Microstructures
- What is the function of a vacuum sintering furnace in the SAGBD process? Optimize Magnetic Coercivity and Performance
- What technological features enhance the efficiency of vacuum furnaces? Boost Performance with Advanced Control & Energy Savings
- What additional processes can a vacuum heat treatment furnace carry out? Unlock Advanced Material Processing
- What role does a vacuum sintering furnace play in the formation of the 'core-rim' structure in Ti(C,N)-FeCr cermets?



















