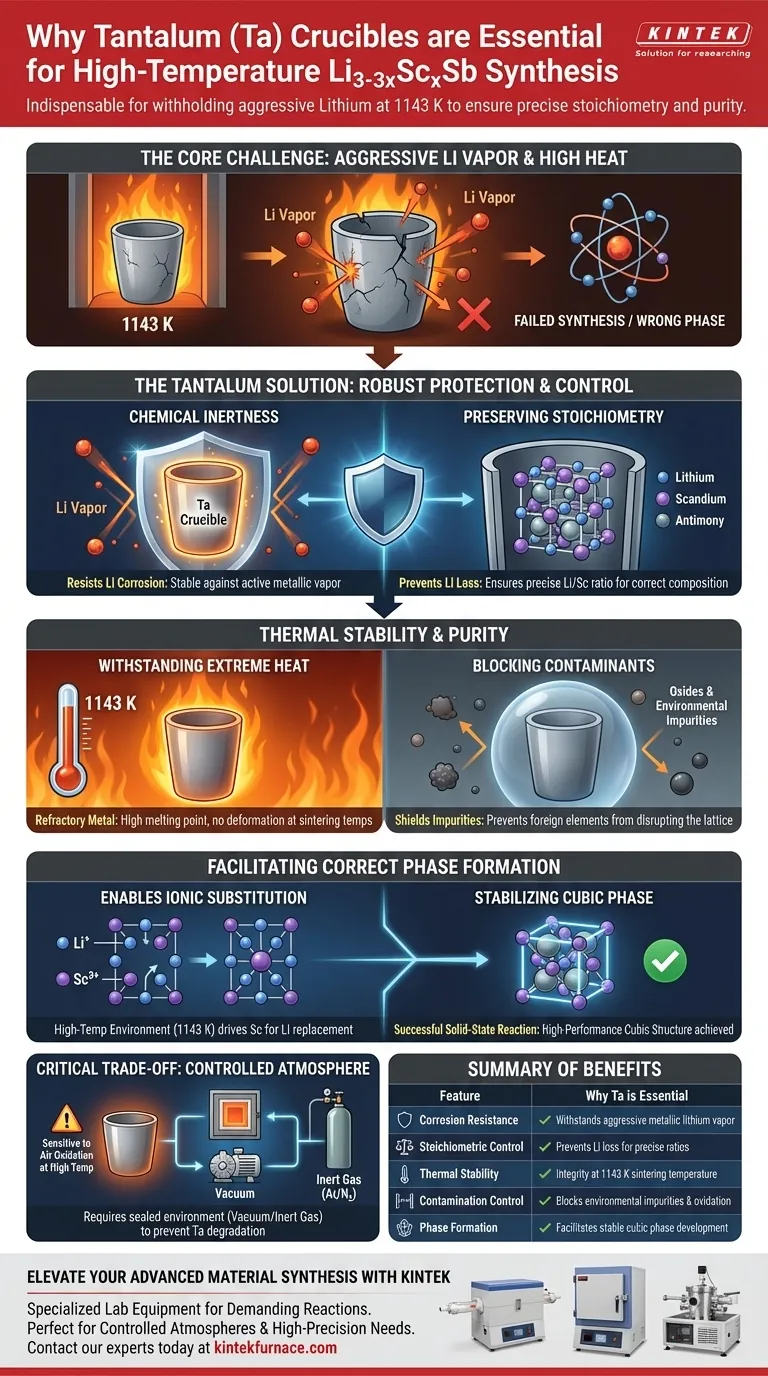
Tantalum (Ta) crucibles are the indispensable choice for synthesizing Li3-3xScxSb due to their unique ability to withstand the aggressive nature of metallic lithium at high temperatures. During the sintering process at 1143 K, the tantalum crucible serves as a chemically inert barrier that prevents the active lithium vapor from corroding the container, while simultaneously blocking environmental impurities from contaminating the sample.
The core challenge in synthesizing Li3-3xScxSb is preventing lithium loss and contamination during the high-energy reaction. Tantalum is essential because it is one of the few materials that maintains structural integrity and chemical inertness against lithium at 1143 K, ensuring the precise stoichiometry required for the material's performance.

The Critical Role of Chemical Inertness
Resisting Lithium Corrosion
Metallic lithium is highly reactive, particularly when vaporized at high temperatures. Most standard crucible materials would degrade or react with the lithium vapor, leading to container failure.
Tantalum possesses superior resistance to this corrosion, remaining stable even when exposed to active metallic lithium vapor. This ensures the physical containment of the synthesis remains intact throughout the process.
Preserving Stoichiometry
For the compound Li3-3xScxSb to form correctly, the ratio of elements must remain exact. If lithium vapor were allowed to react with the crucible or escape, the stoichiometric ratio would shift, resulting in a failed synthesis.
By preventing the loss of active lithium, the tantalum crucible ensures that the chemical composition remains balanced as intended.
Thermal Stability and Purity
Withstanding Extreme Heat
The synthesis of Li3-3xScxSb requires a sintering temperature of 1143 K. This high thermal energy is necessary to drive the solid-state reactions and crystal growth.
Tantalum is a refractory metal with an extremely high melting point, allowing it to endure this environment without softening or deforming.
Blocking Environmental Contaminants
Purity is paramount for the performance of the final material. The tantalum crucible acts as a shield, effectively blocking environmental impurities from entering the reaction zone.
This isolation is critical for preventing the introduction of oxides or other foreign elements that could disrupt the crystal lattice.
Facilitating the Correct Phase Formation
Enabling Ionic Substitution
The high-temperature environment (1143 K) provided by the furnace is what allows Scandium (Sc) ions to successfully replace Lithium (Li) ions.
The crucible's stability ensures this reaction can proceed for the required duration without interruption. This substitution is necessary for the Sc ions to occupy specific tetrahedral vacancies within the lattice.
Stabilizing the Cubic Phase
The ultimate goal of this process is to stabilize the high-performance cubic phase structure of the material.
By maintaining a pure, contaminant-free environment with precise lithium content, the tantalum crucible allows the solid-state reaction to complete successfully, resulting in the desired high-performance structure.
Understanding the Trade-offs
Sensitivity to Oxidation
While Tantalum is excellent at resisting lithium corrosion inside the crucible, Tantalum itself is susceptible to oxidation if exposed to air at these high temperatures.
Operational Requirements
Therefore, the use of a Ta crucible usually necessitates a controlled atmosphere (such as a vacuum or inert gas) within the high-precision tube furnace. Firing a Ta crucible in an oxygen-rich environment would lead to the rapid degradation of the crucible itself.
Making the Right Choice for Your Goal
To ensure the successful synthesis of Li3-3xScxSb, consider the following regarding your experimental setup:
- If your primary focus is stoichiometric accuracy: Rely on the Ta crucible to prevent lithium volatilization; any loss of Li will alter the Li/Sc ratio and degrade performance.
- If your primary focus is phase purity: Ensure the crucible is sealed or utilized within a strictly controlled atmosphere to prevent environmental impurities from breaching the container.
The Tantalum crucible is not just a vessel; it is the primary defense against chemical imbalance, ensuring your high-temperature reaction yields the precise cubic phase structure required.
Summary Table:
| Feature | Why Tantalum (Ta) is Essential |
|---|---|
| Corrosion Resistance | Withstands aggressive metallic lithium vapor at high temperatures. |
| Stoichiometric Control | Prevents lithium loss to maintain precise Li/Sc ratios. |
| Thermal Stability | Refractory properties allow integrity at sintering temperatures of 1143 K. |
| Contamination Control | Acts as a barrier against environmental impurities and oxidation. |
| Phase Formation | Facilitates stable ionic substitution and cubic phase development. |
Elevate Your Advanced Material Synthesis with KINTEK
Precision in high-temperature sintering requires more than just high heat; it requires the right containment environment. KINTEK provides the specialized lab equipment necessary for demanding reactions like Li3-3xScxSb synthesis.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need a controlled atmosphere to protect your Tantalum crucibles or a high-precision furnace customizable for your unique stoichiometric requirements, KINTEK is your partner in laboratory excellence.
Ready to achieve superior phase purity and material performance? Contact our technical experts today to find the perfect thermal solution for your lab.
Visual Guide
References
- Jingwen Jiang, Thomas F. Fässler. Scandium Induced Structural Disorder and Vacancy Engineering in Li<sub>3</sub>Sb – Superior Ionic Conductivity in Li<sub>3−3</sub><i><sub>x</sub></i>Sc<i><sub>x</sub></i>Sb. DOI: 10.1002/aenm.202500683
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the purpose of a PTFE-lined autoclave in TiO2 synthesis? Unlock Precision Nano-material Growth
- What is the function of BN coating in Y2O3-YAM hot-press sintering? Ensure Purity and Smooth Mold Release
- Why is a quartz boat considered an essential carrier tool for the catalytic pyrolysis synthesis of carbon nanotubes?
- Why is ASTM A36 steel plate used for heat treatment furnace frameworks? Reliable Strength & Cost-Efficiency
- Why is it necessary to use a mechanical vacuum pump for SnSe growth? Ensure High-Purity Material Synthesis
- What types of high-temperature laboratory furnace systems are available? Explore 5 Specialized Solutions
- Why are high-purity alumina crucibles selected as the substrate during the sintering of boron-containing stainless steel?
- What are the advantages of nickel crucibles for KOH activation? Ensure High Purity & Thermal Stability up to 700°C



















