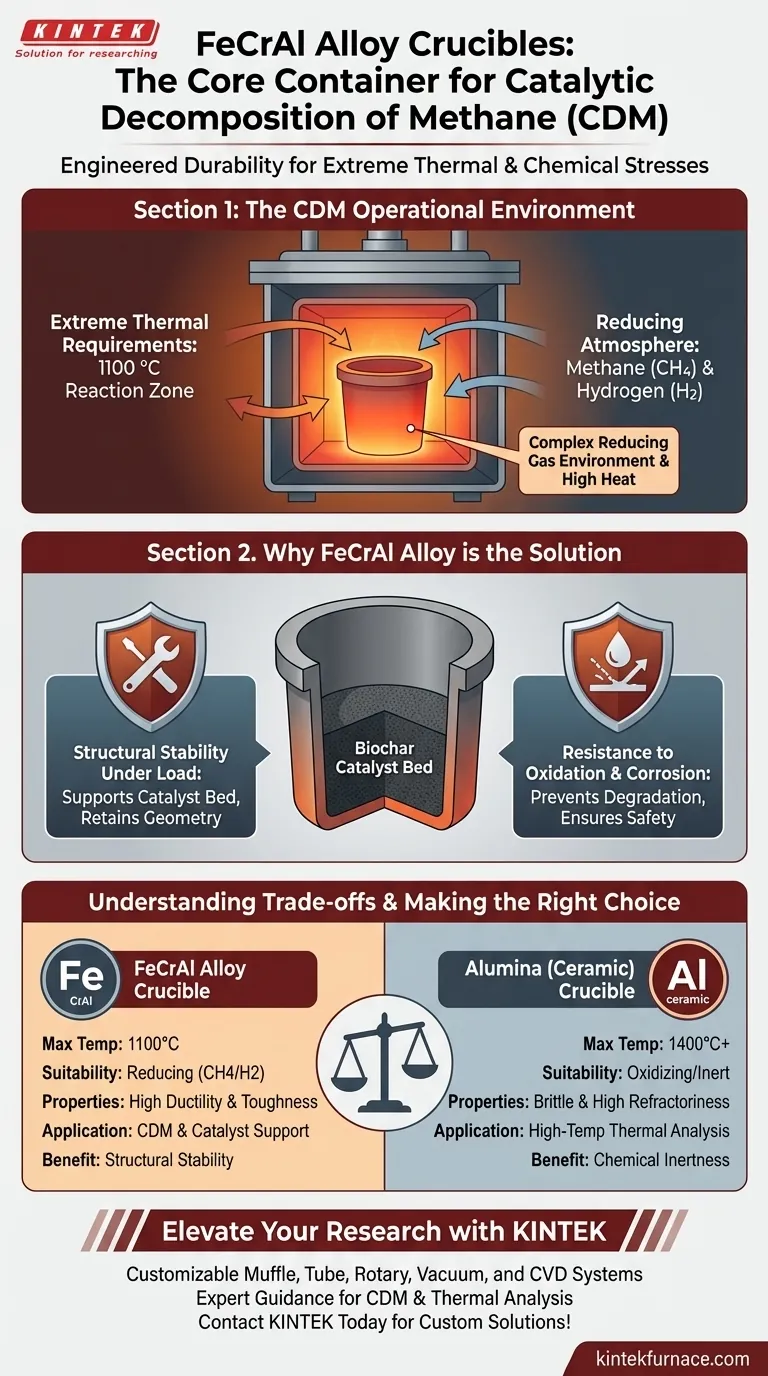
A high-heat-resistant FeCrAl alloy crucible is utilized as the core container in Catalytic Decomposition of Methane (CDM) experiments because it provides the necessary durability to withstand extreme thermal and chemical stresses. It is specifically selected to hold the biochar catalyst bed while maintaining structural integrity at temperatures up to 1100 °C in an aggressive reducing atmosphere.
The primary driver for selecting FeCrAl alloy is its ability to ensure safe, continuous operation by resisting oxidation and corrosion while enduring the complex interaction of methane and hydrogen gases at high heat.

The Operational Environment of CDM
To understand the material choice, you must first understand the harsh conditions inside the reactor.
Extreme Thermal Requirements
CDM processes require high energy to break down methane molecules. The reaction zone often operates at temperatures reaching 1100 °C.
The Reducing Atmosphere
The environment inside the crucible is not just hot; it is chemically active. The presence of methane (CH4) and the production of hydrogen (H2) create a complex reducing gas environment.
This specific atmosphere can degrade standard materials rapidly, stripping away oxide layers that usually protect metals from heat damage.
Why FeCrAl Alloy is the Engineered Solution
FeCrAl (Iron-Chromium-Aluminum) alloys are engineered to solve the specific failure points caused by the CDM environment.
Structural Stability Under Load
The crucible serves a mechanical function: it must physically hold the biochar catalyst bed.
At 1100 °C, many materials soften or warp. FeCrAl retains sufficient mechanical strength to support the catalyst load without deforming, ensuring the geometry of the reaction zone remains consistent.
Resistance to Oxidation and Corrosion
Despite the reducing environment, the alloy is designed to resist oxidation and corrosion.
This resistance is critical for preventing the container wall from degrading, which could lead to a breach, safety hazards, or contamination of the experiment.
Understanding the Trade-offs
While FeCrAl is ideal for CDM, it is important to recognize where its utility ends compared to other materials.
Alloy vs. Ceramic Limits
FeCrAl is a metal alloy chosen for its toughness and specific chemical resistance in reducing gases. However, it has a lower maximum operating temperature compared to ceramics.
For experiments requiring temperatures exceeding 1400 °C or where absolute chemical purity is paramount (such as in thermal analysis of bauxite), alumina crucibles are often preferred. Alumina offers higher refractoriness but lacks the metallic ductility and specific fracture toughness of FeCrAl.
The Cost of Durability
The specialized nature of high-heat-resistant alloys means they are selected for operational safety and longevity over short-term cost. Using a lesser material in a hydrogen-rich, 1100 °C environment would likely lead to rapid embrittlement or structural failure.
Making the Right Choice for Your Goal
Selecting the correct crucible material depends entirely on the chemical atmosphere and temperature range of your specific application.
- If your primary focus is Catalytic Decomposition of Methane (CDM): Choose FeCrAl alloy to ensure structural stability and corrosion resistance in a reducing atmosphere up to 1100 °C.
- If your primary focus is High-Temperature Thermal Analysis: Choose Alumina (ceramic) for superior chemical inertness and stability at temperatures reaching or exceeding 1400 °C.
Match the material properties to your chemical environment to ensure data integrity and operational safety.
Summary Table:
| Feature | FeCrAl Alloy Crucible | Alumina (Ceramic) Crucible |
|---|---|---|
| Max Operating Temp | Up to 1100°C | Up to 1400°C+ |
| Atmosphere Suitability | Reducing (CH4/H2) | Oxidizing/Inert |
| Mechanical Properties | High Ductility & Toughness | Brittle & High Refractoriness |
| Primary Application | CDM & Biochar Catalyst Support | High-Temp Thermal Analysis |
| Key Benefit | Structural Stability Under Load | Chemical Inertness |
Elevate Your Research with Precision Lab Equipment
Choosing the right material is critical for the safety and accuracy of your high-temperature experiments. Backed by expert R&D and manufacturing, KINTEK offers a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized crucibles tailored to your specific process requirements. Whether you are conducting Catalytic Decomposition of Methane (CDM) or high-purity thermal analysis, our lab solutions are fully customizable to meet your unique needs.
Ready to optimize your lab’s performance? Contact KINTEK today for expert guidance and custom solutions!
Visual Guide
References
- Roger Khalil, Øyvind Skreiberg. Catalytic Methane Decomposition for the Simultaneous Production of Hydrogen and Low-Reactivity Biocarbon for the Metallurgic Industry. DOI: 10.3390/en18030558
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of high-alumina ceramic boats? Ensure Purity in I-NC Catalyst Synthesis
- Why are long alumina boats selected as sample containers for zone refining experiments? Optimize Material Purification
- What is the function of a graphite plate in microwave cladding? Ensure Purity & Thermal Uniformity for HEA Synthesis
- How does the vacuum pumping principle of a circulating water vacuum pump differ from jet pumping? Compare Mechanisms & Uses
- Why are high-purity alumina crucibles selected for lithium orthosilicate synthesis? Ensure Purity & Thermal Stability
- What is the function of the circulating water cooling system? Optimize Pyrolysis Oil Condensation and Yield
- Why is a BN coating used in Mg3Sb2 melting? Essential Purity and Protection Guide
- How does the geometric design of a sample basket affect measurement accuracy in thermogravimetric analysis?



















