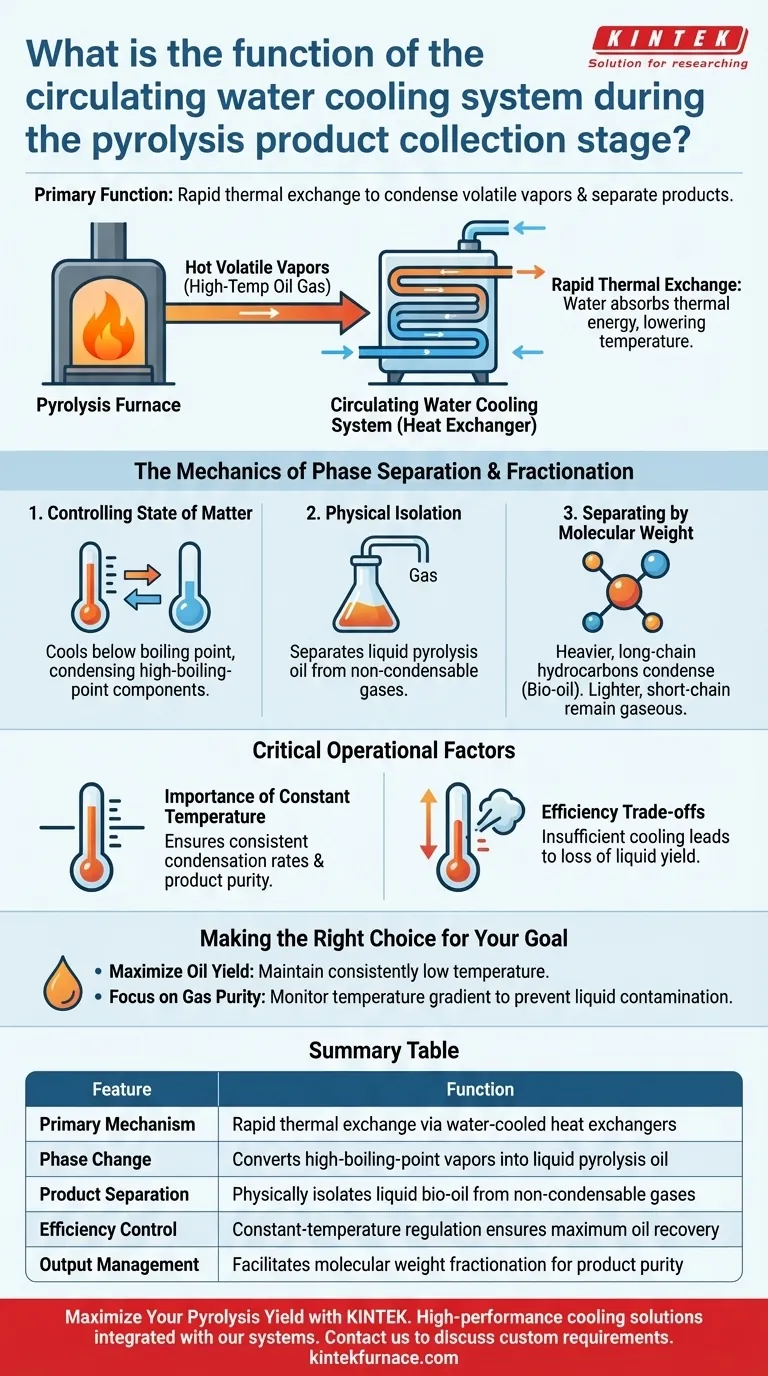
The primary function of the circulating water cooling system is to act as a thermal exchange mechanism that rapidly lowers the temperature of volatile vapors exiting the pyrolysis furnace. By maintaining a constant, lower temperature within the heat exchanger, the system forces a phase change that converts high-boiling-point components into liquid form for collection.
The system acts as the critical separation point between liquid fuel and gaseous byproducts. By controlling thermal conditions, it ensures the efficient condensation of valuable pyrolysis oil while physically isolating non-condensable gases.

The Mechanics of Phase Separation
Rapid Thermal Exchange
The core task of the system is to manage the high-temperature oil gas discharged from the pyrolysis furnace. As these hot vapors enter the cooling pipes, the circulating water absorbs their thermal energy.
Controlling the State of Matter
This rapid cooling lowers the temperature of the heat exchanger below the boiling point of specific compounds. This process efficiently condenses high-boiling-point oil components from a gas phase into a liquid phase.
Physical Isolation of Products
Once condensed, the liquid flows into a collection flask. This creates a physical separation between the liquid products (pyrolysis oil) and the remaining vapors, known as non-condensable gases (pyrolysis gas).
How Fractionation Occurs
Separating by Molecular Weight
While the primary reference focuses on the physical machinery, the process is driven by chemical properties. Heavier, long-chain hydrocarbons have higher boiling points and condense quickly into liquid bio-oil when cooled.
Handling Non-Condensable Gases
Conversely, lighter, short-chain hydrocarbons do not condense at these temperatures. Because they remain in a gaseous state, the system allows them to pass through for separate handling or collection, facilitating a preliminary classification of products.
Critical Operational Factors
The Importance of Constant Temperature
To maximize efficiency, the system typically employs a constant-temperature device. Fluctuations in cooling water temperature can lead to inconsistent condensation rates and impure product collection.
Efficiency Trade-offs
If the cooling capacity is insufficient or the temperature gradient is not steep enough, valuable oil vapors may remain gaseous. This results in a loss of liquid yield as potential oil escapes with the non-condensable gases.
Making the Right Choice for Your Goal
Ideally, your cooling strategy should match your desired product output.
- If your primary focus is maximizing oil yield: Ensure the system maintains a consistently low temperature to force the condensation of all recoverable long-chain hydrocarbons.
- If your primary focus is gas purity: Monitor the temperature gradient to ensure only the lightest short-chain hydrocarbons remain in the gaseous phase, preventing liquid contamination in your gas lines.
The efficiency of your product collection is directly proportional to the stability of your cooling system.
Summary Table:
| Feature | Function in Pyrolysis Collection |
|---|---|
| Primary Mechanism | Rapid thermal exchange via water-cooled heat exchangers |
| Phase Change | Converts high-boiling-point vapors into liquid pyrolysis oil |
| Product Separation | Physically isolates liquid bio-oil from non-condensable gases |
| Efficiency Control | Constant-temperature regulation ensures maximum oil recovery |
| Output Management | Facilitates molecular weight fractionation for product purity |
Maximize Your Pyrolysis Yield with KINTEK
Precise thermal management is the difference between high-quality bio-oil and lost revenue. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance cooling solutions integrated with our Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need a standard setup or a fully customizable lab high-temp furnace, our technology ensures stable condensation and superior product separation.
Ready to optimize your pyrolysis process? Contact KINTEK today to discuss your custom furnace and cooling requirements with our engineering team.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a laboratory vacuum drying oven preferred for Al2O3/TiC/SiC(w) mixed slurries? Prevent Oxidation & Agglomeration
- What is the importance of using spot-welded K-type thermocouples in DP steel heat treatment? Master Thermal Precision
- Why is a vacuum drying oven essential for Pd-Ni/ZrO2 catalyst preparation? Ensure Uniform Metal Distribution
- What role do refractory bricks and graphite paper play within a quartz tube? Optimize RuMoOx/NC Synthesis Efficiency
- Why is a graphite crucible used for melting Al-Mg-Si alloys? Superior Purity & Thermal Efficiency
- What are the benefits of a vacuum chamber? Achieve Unmatched Process Control and Purity
- Why is laboratory heating equipment critical for photothermal actuators? Master Structural Curing & Precision Thermal Control
- What is the necessity of quartz vacuum sealing for BiCuSeO? Protect Phase Purity and Prevent Selenium Volatilization



















