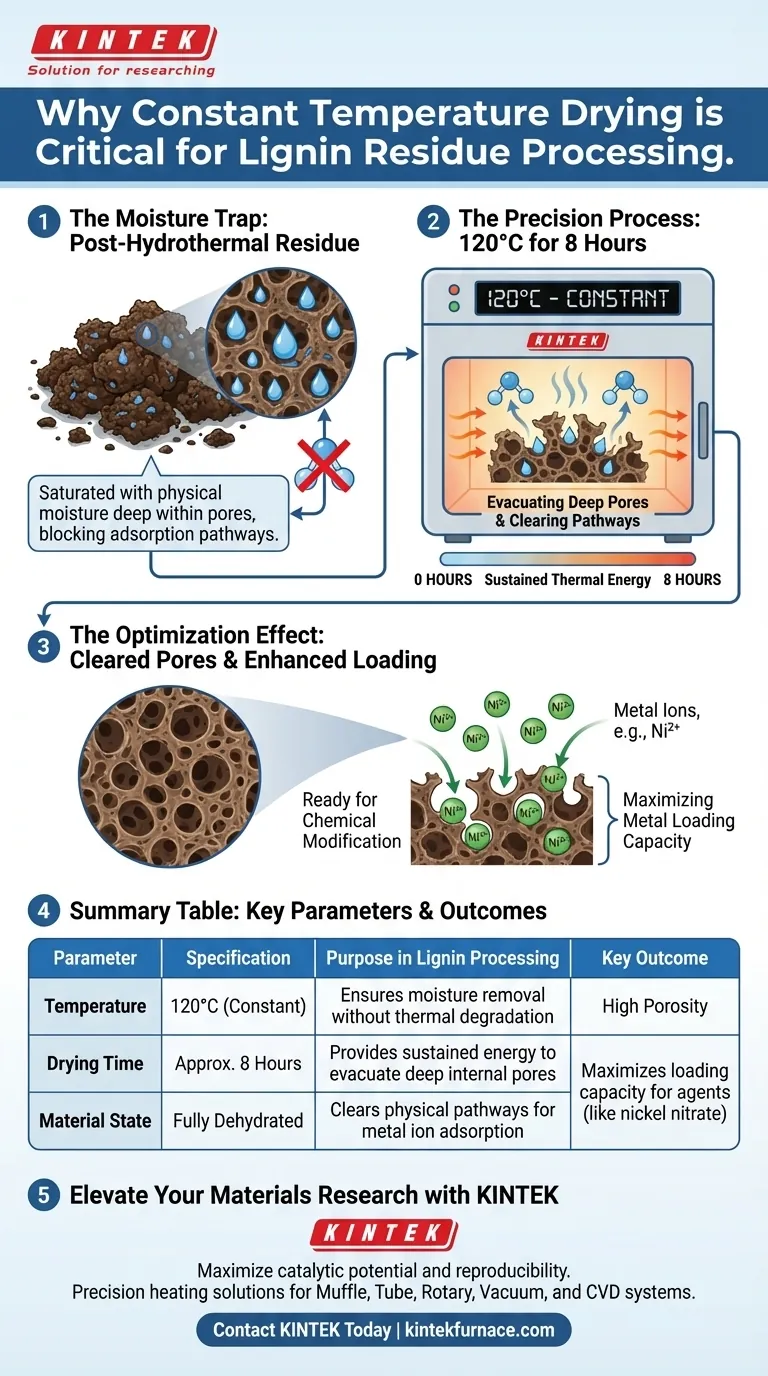
A constant temperature drying oven is critical for preparing lignin residue for chemical modification. Specifically, it is required to completely remove the physical moisture that permeates the material during hydrothermal treatment and filtration. By subjecting the residue to 120°C for approximately 8 hours, you ensure the material is thoroughly dehydrated, which is a prerequisite for effective downstream processing.
Complete dehydration is not merely about drying the material; it is about evacuating the internal pore structure. If moisture remains in these pores, it physically blocks the adsorption of metal ions in subsequent steps, compromising the material's final performance.

The Mechanics of Moisture Removal
Eliminating Saturation
Following hydrothermal treatment and filtration, the lignin residue is saturated with water. This moisture is not just on the surface but trapped deep within the physical matrix of the residue.
The Necessity of Sustained Heat
A quick dry is insufficient for this type of porous material. Processing at 120°C for 8 hours provides the sustained thermal energy required to drive out water molecules trapped in the intricate structure without degrading the lignin itself.
Optimizing the Porous Structure
Clearing the Pathways
The value of the lignin residue often lies in its porosity. The drying process functions as a "reset," clearing water from these pores to create accessible void space.
Preparing for Adsorption
Once the pores are cleared of water, the precursor is ready to interact with other chemical agents. The open pore structure is essential for the material to act as an effective host for metal ions.
Enhancing Metal Loading Capacity
Increasing Ion Uptake
The primary goal of this drying stage is to maximize the material's ability to adsorb metal ions, such as those found in nickel nitrate solutions. A dry, open pore structure absorbs these solutions much more effectively than a moist one.
Maximizing Performance
Thorough dehydration directly correlates to increased metal loading capacity. By ensuring the pores are empty, you allow for a higher concentration of metal ions to be anchored onto the lignin precursor.
Operational Considerations and Risks
The Cost of Incomplete Drying
If the drying time is shortened or the temperature fluctuates, residual moisture will likely remain in the deepest pores. This water competes with the metal solution for space, significantly reducing the efficiency of the metal loading process.
Temperature Precision
While removing water is vital, temperature control is equally important. The oven must maintain a constant temperature to ensure uniform drying without subjecting the organic lignin structure to thermal shock or degradation that might occur at significantly higher temperatures.
Ensuring Process Integrity
To maximize the quality of your lignin residue precursor, strictly adhere to the drying parameters.
- If your primary focus is maximizing catalytic potential: Ensure the full 8-hour drying cycle is completed to guarantee that maximum pore volume is available for metal ion adsorption.
- If your primary focus is process reproducibility: Monitor the oven to ensure it holds a steady 120°C, preventing batch-to-batch variations in moisture content and pore accessibility.
Proper dehydration is the invisible foundation that determines the success of subsequent chemical functionalization.
Summary Table:
| Parameter | Specification | Purpose in Lignin Processing |
|---|---|---|
| Temperature | 120°C (Constant) | Ensures moisture removal without thermal degradation |
| Drying Time | Approx. 8 Hours | Provides sustained energy to evacuate deep internal pores |
| Material State | Fully Dehydrated | Clears physical pathways for metal ion adsorption |
| Key Outcome | High Porosity | Maximizes loading capacity for agents like nickel nitrate |
Elevate Your Materials Research with KINTEK
Maximize the catalytic potential and reproducibility of your lignin precursors with precision heating solutions. Backed by expert R&D and manufacturing, KINTEK offers a wide range of customizable lab equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically designed to meet the rigorous demands of high-temperature material processing.
Don't let residual moisture compromise your results. Whether you are optimizing pore structures or enhancing metal loading capacity, our high-performance furnaces provide the temperature stability your research requires.
Ready to optimize your lab's thermal processing? Contact KINTEK today to discuss your unique project needs and discover the difference precision makes.
Visual Guide
References
- Sunshine D. Kurbah, Ndege Simisi Clovis. Lignocellulosic Biomass Derived Carbon Supported Nickel Nanoparticles as an Efficient Catalyst for Reduction of Nitroarenes. DOI: 10.17807/orbital.v16i4.21957
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the significance of pre-equilibrating samples in silicate studies? Maximize Experimental Efficiency
- Why is thermal insulation applied to cylindrical components in thermal stress tests? Enhance Calculation Precision
- Why are User-Defined Functions (UDFs) necessary for modeling complex combustion? Unlock Precision in Furnace Simulation
- What is the function of ball milling in Li-NASICON synthesis? Optimize Your Solid Electrolyte Performance
- What is the purpose of high-purity argon in Fe60Co10-xNi15Cr15Six alloy preparation? Ensure Purity for Laser Cladding
- What role does the high-temperature boiling step play in rice husk silica conversion? Boost Your Extraction Yields
- What is the purpose of pre-baking sapphire substrates? Master Atomic Flatness for Superior Thin Film Growth
- What is the function of a high-temperature heating reactor in OPF delignification? Unlock High-Purity Cellulose



















