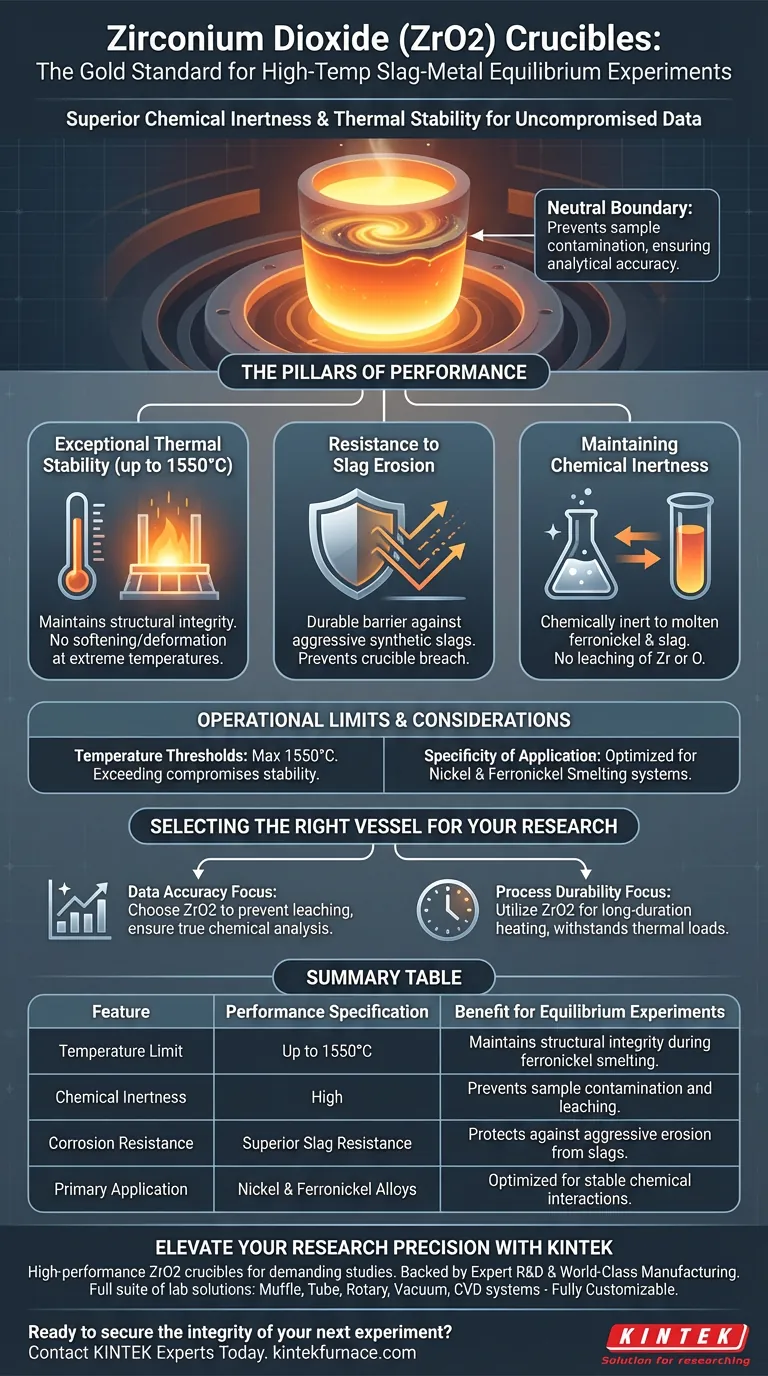
Zirconium Dioxide (ZrO2) crucibles are the vessel of choice for high-temperature slag-metal equilibrium experiments primarily due to their superior chemical inertness and thermal stability. They are specifically engineered to withstand extreme environments, such as nickel alloy smelting at temperatures up to 1550°C, without reacting with the molten contents or degrading over long durations.
In equilibrium experiments, the purity of the sample is paramount. Zirconium Dioxide crucibles act as a neutral boundary, preventing the vessel material from dissolving into the molten alloy and contaminating the data, thereby ensuring the analytical results represent the true chemical state of the sample.
The Pillars of Reaction Vessel Performance
To understand why ZrO2 is utilized, one must look at the specific physical and chemical demands placed on a crucible during slag-metal equilibrium studies.
Exceptional Thermal Stability
Equilibrium experiments often require maintaining high temperatures for extended periods to allow chemical reactions to stabilize.
Zirconium Dioxide exhibits remarkable stability at temperatures up to 1550°C. Unlike lesser refractory materials, it maintains its structural integrity and does not soften or deform under the intense heat required for smelting ferronickel alloys.
Resistance to Slag Erosion
Synthetic slags used in these experiments are highly corrosive and can rapidly eat away at standard crucible linings.
ZrO2 crucibles offer superior resistance to slag erosion. They create a durable barrier against the aggressive chemical attack of the molten slag, ensuring the crucible does not breach or degrade before the experiment is complete.
Maintaining Chemical Inertness
The most critical factor in equilibrium studies is preventing cross-contamination between the containment vessel and the sample.
ZrO2 is utilized because it remains chemically inert regarding the molten ferronickel and slag. It effectively contains the melt without leaching zirconium or oxygen into the alloy, which is essential for obtaining accurate, uncontaminated analytical results.
Operational Limits and Considerations
While Zirconium Dioxide is a robust material, successful application requires adhering to its operational parameters.
Temperature Thresholds
It is vital to note the specific thermal limit of 1550°C. While highly effective up to this point, exceeding this temperature threshold could compromise the crucible’s structural stability or resistance properties.
Specificity of Application
The material is specifically highlighted for its effectiveness in nickel alloy and ferronickel smelting. While its properties are generally robust, its performance is optimized for the specific chemical interactions found in these slag-metal systems.
Selecting the Right Vessel for Your Research
When designing your experimental setup, your choice of crucible should align with your specific analytical goals.
- If your primary focus is Data Accuracy: Choose ZrO2 to prevent crucible constituent leaching and ensure that your final chemical analysis reflects only the interaction between the slag and the metal.
- If your primary focus is Process Durability: Utilize ZrO2 for experiments requiring long-duration heating cycles up to 1550°C, as it withstands the physical stress of prolonged thermal loads.
By selecting Zirconium Dioxide, you prioritize the integrity of your equilibrium data through material stability and chemical neutrality.
Summary Table:
| Feature | Performance Specification | Benefit for Equilibrium Experiments |
|---|---|---|
| Temperature Limit | Up to 1550°C | Maintains structural integrity during ferronickel smelting. |
| Chemical Inertness | High | Prevents sample contamination and leaching of crucible materials. |
| Corrosion Resistance | Superior Slag Resistance | Protects against aggressive erosion from synthetic slags. |
| Primary Application | Nickel & Ferronickel Alloys | Optimized for stable chemical interactions in metallic systems. |
Elevate Your Research Precision with KINTEK
Don’t let crucible contamination compromise your analytical data. KINTEK provides high-performance Zirconium Dioxide crucibles designed for the most demanding slag-metal equilibrium studies. Backed by expert R&D and world-class manufacturing, we offer a full suite of lab high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique experimental needs.
Ready to secure the integrity of your next experiment? Contact KINTEK Experts Today
Visual Guide
References
- Erdenebold Urtnasan, Jei‐Pil Wang. Artificial Slags with Modulated Properties for Controlled Nickel Dissolution in Smelting Process. DOI: 10.1007/s12666-024-03304-0
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What is the function of a high-precision mass flow controller (MFC) in CdS nanobelt vapor deposition?
- Why are graphite crucible furnaces used in vacuum or protective atmosphere environments? Prevent Oxidation and Ensure Purity
- What are the risks of using high-purity alumina crucibles for periodate decomposition? Avoid Crucial Data Errors
- Where are water circulating vacuum pumps commonly used? Essential for Lab and Industrial Vapor Handling
- What is the function of a vacuum system in PLD? Ensure High-Density, Pure Electrolyte Thin Films
- Why is a vacuum pumping system essential for DD6 alloy and ceramic shell experiments? Achieve High-Purity Results
- What are the advantages of using a laboratory vacuum drying oven for modified ZnO nanomaterials? Protect Nano-Integrity
- What role does a mass flow controller (MFC) play in plasma-based aluminum reduction? Precision Control for High Yields



















