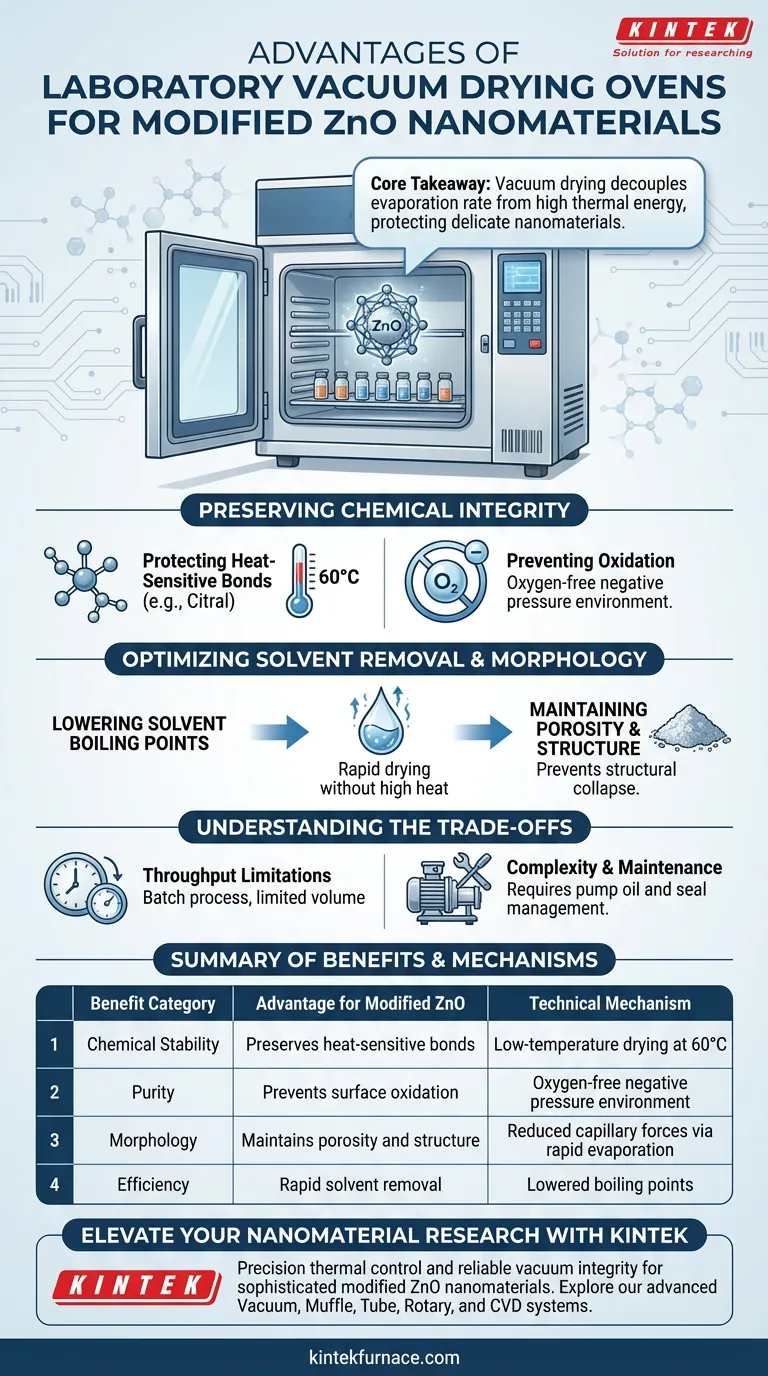
The primary advantage of using a laboratory vacuum drying oven for modified ZnO nanomaterials is the preservation of heat-sensitive chemical bonds through low-temperature processing. By creating a negative pressure environment, the oven significantly lowers the boiling point of cleaning solvents. This allows for rapid, thorough drying at a moderate 60°C, preventing the thermal degradation of delicate citral molecules and ensuring the material's pH-responsive performance remains intact.
Core Takeaway Vacuum drying decouples the evaporation rate from high thermal energy, allowing you to remove stubborn solvents without subjecting delicate nanomaterials to destructive heat. This process protects the chemical functionality of surface modifications while preventing oxidation.

Preserving Chemical Integrity
The most critical challenge in processing modified nanomaterials is removing solvents without destroying the surface modification itself.
Protecting Heat-Sensitive Bonds
Modified ZnO often incorporates organic molecules, such as citral, to achieve specific functionalities like pH responsiveness. These molecules are inherently heat-sensitive.
Subjecting these materials to standard high-temperature drying can degrade the covalent bonds linking the modifier to the nanoparticle. By utilizing a vacuum oven, you can operate at approximately 60°C, a temperature safe for these organic bonds, while still achieving rapid solvent evaporation.
Preventing Oxidation
Standard drying ovens expose materials to heated air, which can accelerate oxidative reactions.
A vacuum environment effectively removes oxygen from the chamber. This prevents the potential oxidation of the nanomaterial surface or the organic modifier, ensuring the chemical composition remains stable throughout the drying phase.
Optimizing Solvent Removal and Morphology
Beyond chemical preservation, the physical mechanism of vacuum drying offers distinct structural advantages for nanomaterials.
Lowering Solvent Boiling Points
The vacuum environment reduces the atmospheric pressure surrounding the sample. This physical change significantly lowers the boiling point of common solvents like water or ethanol.
This allows solvents to evaporate vigorously at temperatures far below their standard boiling points. You achieve a "hard dry" without the "hard heat" that would typically cause phase changes or structural damage.
Maintaining Porosity and Structure
While the primary goal for modified ZnO is protecting chemical bonds, vacuum drying also aids in preserving physical morphology.
Rapid evaporation under vacuum helps prevent the structural collapse often caused by prolonged exposure to capillary forces during slow air drying. This helps maintain the loose, porous nature of the powder, which is essential for subsequent processing steps like grinding or molding.
Understanding the Trade-offs
While vacuum drying is superior for heat-sensitive nanomaterials, it introduces specific operational considerations compared to standard thermal drying.
Throughput Limitations
Vacuum drying is inherently a batch process. Unlike conveyor-belt or continuous air dryers, the chamber must be sealed, evacuated, and repressurized for every cycle. This limits the volume of material you can process in a given timeframe.
Complexity and Maintenance
The system requires a vacuum pump and perfectly sealed chambers to function. This introduces variables such as pump oil maintenance, seal integrity, and the management of condensed solvent vapors, which requires more operator attention than a simple convection oven.
Making the Right Choice for Your Goal
To ensure you are applying this technology correctly to your project, consider the following specific recommendations.
- If your primary focus is Functional Performance: Prioritize the vacuum level to keep temperatures at or below 60°C, ensuring the pH-responsive citral modification remains chemically active.
- If your primary focus is Structural Morphology: Use the vacuum to accelerate solvent removal, preventing capillary collapse and ensuring a loose, porous powder structure.
- If your primary focus is Purity: Rely on the vacuum environment to exclude oxygen, preventing surface oxidation that could interfere with electrochemical or catalytic properties.
By substituting pressure reduction for thermal intensity, you ensure the sophisticated chemistry of your modified nanomaterials survives the processing stage intact.
Summary Table:
| Benefit Category | Advantage for Modified ZnO | Technical Mechanism |
|---|---|---|
| Chemical Stability | Preserves heat-sensitive bonds (e.g., Citral) | Low-temperature drying at 60°C |
| Purity | Prevents surface oxidation | Oxygen-free negative pressure environment |
| Morphology | Maintains porosity and structure | Reduced capillary forces via rapid evaporation |
| Efficiency | Rapid solvent removal | Lowered boiling points of water/ethanol |
Elevate Your Nanomaterial Research with KINTEK
Precision is paramount when processing sophisticated modified ZnO nanomaterials. At KINTEK, we understand that protecting heat-sensitive chemical functionality requires superior thermal control and reliable vacuum integrity.
Backed by expert R&D and manufacturing, KINTEK offers advanced Vacuum, Muffle, Tube, Rotary, and CVD systems, all customizable to meet your unique laboratory requirements. Whether you are preserving pH-responsive citral bonds or preventing surface oxidation, our high-temp furnaces provide the stable, controlled environments your research demands.
Ready to optimize your drying process? Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Yanan Fan, Yongheng Zhu. Research on pH-responsive antibacterial materials using citral-modified zinc oxide nanoparticles. DOI: 10.1093/fqsafe/fyae010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What is the function of an alumina boat during high-temperature activation of porous carbon? Durable Lab Solutions
- What are the technical advantages of using quartz tubes for fiber optic sensors? Optimize High-Temp Performance
- What is the role of an optical pyrometer in diffusion bonding? Ensure Precision in High-Temperature Simulations
- How do the quartz crucible and descending device function in Bridgman method? Precision Growth for CsPbBr3 Crystals
- How are constant temperature water baths and drying ovens utilized to verify bonding quality? Master EN 314-1 Testing
- Why are high-purity alumina grinding balls used for Al2O3/TiC milling? Master Chemical Consistency
- Why is the integration of a K-type thermocouple and a data logger necessary for Vanadis 60 steel? Unlock Precision.
- Why is Beryllium Oxide (BeO) used as a viscometer crucible? Superior Stability for High-Temperature Alloy Research



















