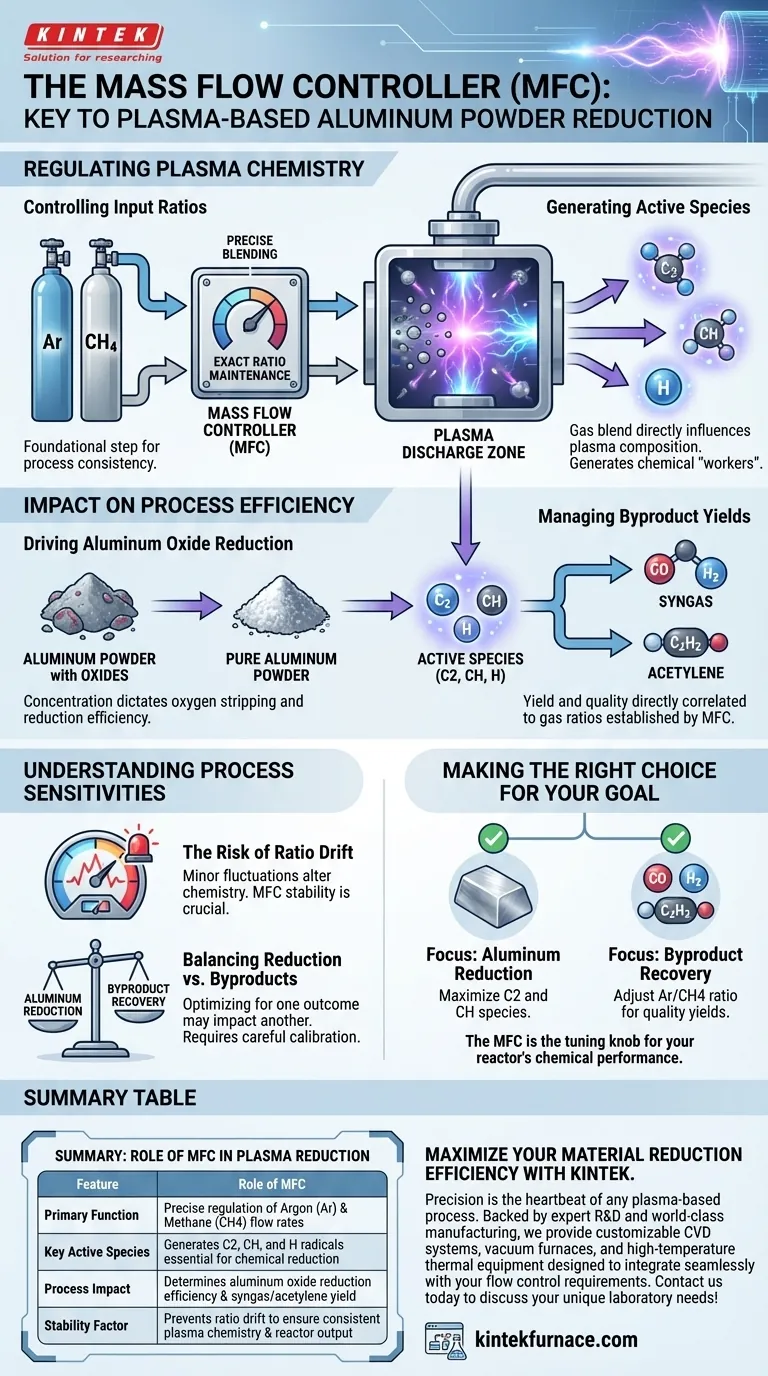
In the plasma-based reduction of aluminum powder, the Mass Flow Controller (MFC) serves as the critical regulator of the reaction environment. Its primary function is to maintain precise flow rates and specific ratios of input gases, specifically argon (Ar) and methane (CH4), entering the system.
The MFC does more than just move gas; it dictates the chemistry of the plasma. By strictly controlling input ratios, the MFC determines the concentration of active species, which drives both the efficiency of aluminum oxide reduction and the yield of valuable byproducts.

Regulating the Plasma Chemistry
The reduction of aluminum powder is a highly sensitive chemical process. The MFC ensures that the conditions within the plasma discharge zone remain optimal for reaction.
Controlling Input Ratios
The MFC is responsible for the precise blending of the carrier gas, argon (Ar), and the reactive gas, methane (CH4).
Maintaining the exact ratio between these two gases is the foundational step of the entire process.
Generating Active Species
The specific blend of gases regulated by the MFC directly influences the composition of the plasma.
Proper flow control facilitates the generation of critical active species, including C2, CH, and H. These species are the chemical "workers" that actually perform the reduction.
Impact on Process Efficiency
The settings applied to the Mass Flow Controller have a downstream effect on the final output of the reactor. The relationship is linear: flow control dictates plasma composition, which dictates results.
Driving Aluminum Oxide Reduction
The concentration of active species (C2, CH, H) determines how effectively oxygen is stripped from the aluminum powder.
If the MFC maintains optimal reactant levels, the reduction efficiency of aluminum oxide is maximized.
Managing Byproduct Yields
The process produces secondary outputs, specifically syngas and acetylene.
The yield and quality of these byproducts are directly correlated to the gas ratios established by the MFC.
Understanding Process Sensitivities
While the MFC enables precision, it also highlights the vulnerability of the process. Understanding the trade-offs of flow control is essential for consistent results.
The Risk of Ratio Drift
Because the process relies on specific active species (C2, CH, H), even minor fluctuations in gas flow can alter the plasma chemistry.
If the MFC fails to maintain strict stability, the concentration of these species will drop, leading to incomplete reduction of the aluminum oxide.
Balancing Reduction vs. Byproducts
Optimizing for one outcome may impact another.
A flow ratio designed to maximize syngas production might differ slightly from the ratio needed for maximum acetylene yield, requiring careful calibration of the MFC based on your primary goal.
Making the Right Choice for Your Goal
To maximize the effectiveness of your plasma-based reduction system, you must align your MFC settings with your specific objectives.
- If your primary focus is Aluminum Reduction: Calibrate the MFC to maximize the concentration of C2 and CH species, as these directly drive the removal of oxides.
- If your primary focus is Byproduct Recovery: Adjust the Argon/Methane ratio to favor the formation of species that recombine into high-quality syngas or acetylene.
The Mass Flow Controller is not just a valve; it is the tuning knob for the entire chemical performance of your reactor.
Summary Table:
| Feature | Role of MFC in Plasma Reduction |
|---|---|
| Primary Function | Precise regulation of Argon (Ar) and Methane (CH4) flow rates |
| Key Active Species | Generates C2, CH, and H radicals essential for chemical reduction |
| Process Impact | Determines aluminum oxide reduction efficiency and syngas/acetylene yield |
| Stability Factor | Prevents ratio drift to ensure consistent plasma chemistry and reactor output |
Maximize Your Material Reduction Efficiency with KINTEK
Precision is the heartbeat of any plasma-based process. At KINTEK, we understand that even minor fluctuations in gas flow can compromise your results. Backed by expert R&D and world-class manufacturing, we provide high-performance laboratory solutions, including customizable CVD systems, vacuum furnaces, and high-temperature thermal equipment designed to integrate seamlessly with your flow control requirements.
Whether you are optimizing aluminum reduction or targeting specific byproduct yields, our team is ready to build the custom high-temp system your research demands. Contact us today to discuss your unique laboratory needs and explore our range of customizable furnace systems!
Visual Guide
References
- Alexander Logunov, Sergey S. Suvorov. Plasma–Chemical Low-Temperature Reduction of Aluminum with Methane Activated in Microwave Plasma Discharge. DOI: 10.3390/met15050514
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Vacuum Induction Melting Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
People Also Ask
- What roles do high-purity graphite molds play during the Spark Plasma Sintering (SPS) of Ba0.95La0.05FeO3-δ? Essential Guide
- Why are fume hoods and sealed quartz tubes mandatory for BiF3 and SbF3? Safety in High-Temp Fluoride Reactions
- What is the function of a vacuum ampoule during the synthesis of ZnGeP2? Ensure Purity and Chemical Stability
- What are the typical size ranges available for quartz tubes used in laboratory furnaces? Find Your Perfect Fit for High-Temp Applications
- What is the role of specialized sealing ferrules in heating experiments? Ensure Hermetic Isolation and Purity
- What is the function of a laboratory electric thermostatic drying oven in ZIF-8/ZIF-67 prep? Ensure MOF Integrity
- How does the use of laboratory grinding equipment benefit NRBBO:Eu2+ phosphors? Optimize Your Material Synthesis
- What is the primary function of a radiation pyrometer in validating furnace simulations? Ensure Model Accuracy



















