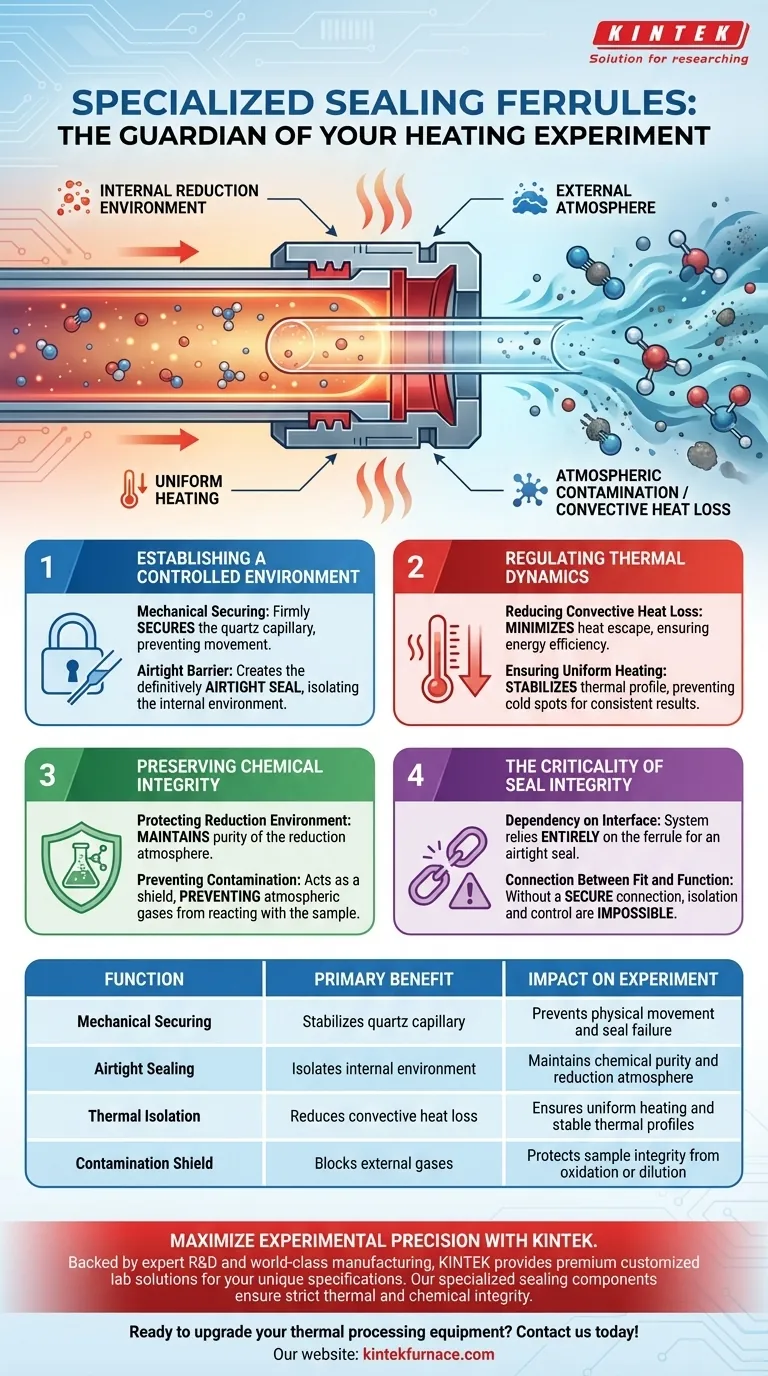
Specialized sealing ferrules act as the critical barrier that isolates your experimental setup from the outside world. Their primary function is to physically secure the quartz capillary while creating an airtight seal that strictly separates the internal reduction environment from external atmospheric conditions.
The ferrule is the linchpin of experimental integrity. By creating an airtight seal, it simultaneously stabilizes the thermal profile by reducing convective heat loss and preserves chemical purity by blocking external contaminants.

Establishing a Controlled Environment
Securing the Capillary
The foundational role of the ferrule is mechanical. It must firmly secure the quartz capillary within the experimental apparatus.
Without this secure attachment, the capillary is vulnerable to movement, which jeopardizes the stability of the entire setup.
Creating an Airtight Barrier
Once secured, the ferrule performs its most critical function: establishing an airtight seal.
This seal is the definitive boundary that isolates the internal reduction environment from the external laboratory atmosphere. Without this isolation, control over the experiment is impossible.
Regulating Thermal Dynamics
Reducing Convective Heat Loss
Temperature consistency is often threatened by uncontrolled airflow or heat escape.
By sealing the system, these ferrules significantly reduce convective heat loss during the heating process. This ensures that the energy input is directed efficiently into the sample rather than escaping into the surroundings.
Ensuring Uniform Heating
The reduction of heat loss leads to a more stable thermal environment.
This thermal isolation ensures uniform heating of the sample. By preventing cold spots or fluctuations caused by convection, the ferrule helps guarantee that the entire sample undergoes the same thermal history.
Preserving Chemical Integrity
Protecting the Reduction Environment
In reduction experiments, the specific chemical composition of the internal atmosphere is paramount.
The ferrule ensures that the purity of the reduction atmosphere is maintained throughout the experiment.
Preventing Contamination
The airtight nature of the seal acts as a shield against the outside world.
It protects the sample integrity by strictly preventing atmospheric contamination. This ensures that no external gases react with the sample or dilute the reduction environment.
The Criticality of Seal Integrity
Dependency on the Interface
While the ferrule is a small component, the success of the experiment relies heavily on its performance.
There is no redundancy here; the system relies entirely on the ferrule to provide an airtight seal. If this seal is compromised, you simultaneously lose thermal control and chemical purity.
The Connection Between Fit and Function
The ability to isolate the environment is directly tied to how well the ferrule secures the capillary.
If the component fails to secure the quartz capillary properly, the airtight seal will inevitably fail. You cannot have isolation without a mechanically secure connection.
Ensuring Experimental Success
To achieve reliable data, you must ensure your sealing components are functioning correctly.
- If your primary focus is Thermal Consistency: Ensure the seal is airtight to minimize convective heat loss, which is the primary driver of non-uniform heating.
- If your primary focus is Sample Purity: Verify the ferrule firmly secures the capillary to prevent atmospheric contamination from compromising the reduction environment.
The specialized ferrule is not just a connector; it is the guardian of your experiment's thermal and chemical variables.
Summary Table:
| Function | Primary Benefit | Impact on Experiment |
|---|---|---|
| Mechanical Securing | Stabilizes quartz capillary | Prevents physical movement and seal failure |
| Airtight Sealing | Isolates internal environment | Maintains chemical purity and reduction atmosphere |
| Thermal Isolation | Reduces convective heat loss | Ensures uniform heating and stable thermal profiles |
| Contamination Shield | Blocks external gases | Protects sample integrity from oxidation or dilution |
Maximize Experimental Precision with KINTEK
Don’t let a weak seal compromise your high-temperature research. Backed by expert R&D and world-class manufacturing, KINTEK provides premium lab solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to your unique specifications. Our specialized sealing components ensure your quartz capillaries maintain the strict thermal and chemical integrity required for sensitive reduction experiments.
Ready to upgrade your thermal processing equipment? Contact us today to discuss your project with our engineering team.
Visual Guide
References
- Yuzhao Wang, Samuli Urpelainen. In Situ SXRD Study of Phase Transformations and Reduction Kinetics in Iron Ore During Hydrogen-Based High-Temperature Reduction. DOI: 10.1007/s11663-025-03725-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What function does a laboratory blast drying oven perform? Optimize Pretreatment for Magnetic Particles
- How does a high-precision analog pressure gauge contribute to the gas delivery system in magnesium combustion experiments?
- What are the primary functions of the vacuum pump system and inert gases? Achieve High-Purity Atomization
- What is the role of a mechanical vacuum pump in the preparation of FeAl alloys? Achieve 10⁻² Pa for Pure Synthesis
- Why are zirconia grinding jars and milling balls ideal for Bismuth Telluride? Achieve 200nm Purity and Performance
- What characteristics are required for reaction vessels in PI-COFs synthesis? Ensure High-Pressure Safety and Purity
- What is the necessity of using a laboratory vacuum drying oven when processing Fe-N-C catalyst powders?
- What maintenance is required after using the alumina furnace tube? Ensure Longevity and Purity in Your Lab



















