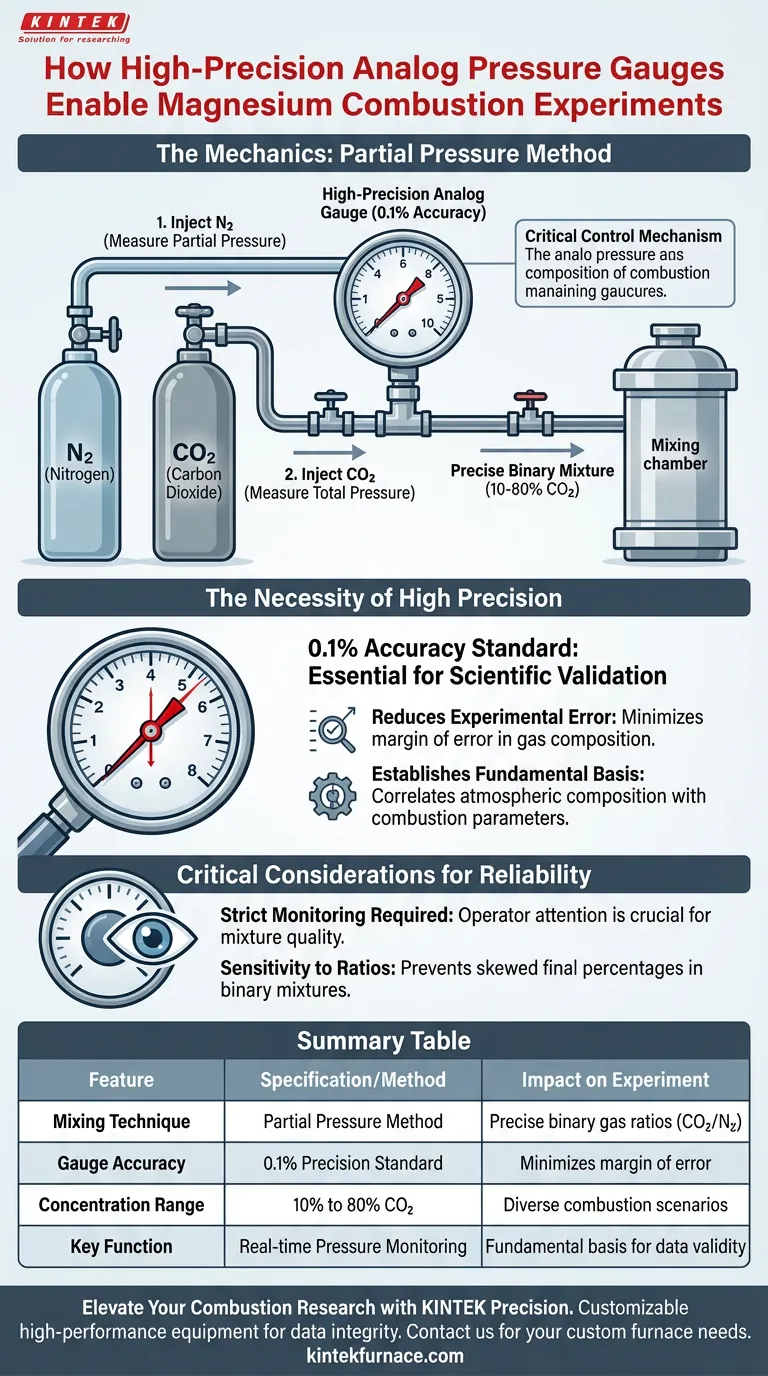
A high-precision analog pressure gauge acts as the critical control mechanism for establishing the specific environmental conditions required in magnesium combustion experiments. By utilizing a technique known as the partial pressure method, this instrument enables researchers to meticulously construct binary gas mixtures—specifically Carbon Dioxide (CO2) and Nitrogen (N2)—with exacting ratios.
The gauge functions as the definitive standard for mixture accuracy; without its strict monitoring of individual gas injection, reliable data regarding how atmospheric composition affects combustion cannot be generated.

The Mechanics of Gas Mixture Preparation
Utilizing the Partial Pressure Method
The gas delivery system relies on the partial pressure method to create the test atmosphere. This involves injecting gases into the chamber sequentially rather than simultaneously.
Monitoring Individual Components
The high-precision gauge measures the pressure of the first gas (e.g., Nitrogen) as it enters the system. Once the specific target pressure is reached, the second gas (e.g., CO2) is added until the total pressure corresponds to the desired ratio.
Achieving Specific Concentrations
This method allows for the creation of highly variable environments. Researchers can produce accurate concentrations ranging from 10% to 80% CO2, allowing for a wide spectrum of combustion scenarios to be tested.
The Necessity of High Precision
The 0.1% Accuracy Standard
Standard industrial gauges are often insufficient for scientific validation. These experiments utilize analog gauges with 0.1% accuracy.
Reducing Experimental Error
Because the ratio of gases is the independent variable in these experiments, any deviation in pressure reading corrupts the results. A 0.1% accuracy rating minimizes the margin of error in the gas composition.
Establishing the Fundamental Basis
The validity of the entire experiment rests on the gas mixture. Accurate pressure readings serve as the fundamental basis for correlating changes in atmospheric composition with changes in combustion parameters.
Critical Considerations for Reliability
Strict Monitoring Requirements
The primary reference highlights the need for strictly monitoring the pressure. Because the system relies on an analog readout, the quality of the mixture is directly tied to the operator's attention to the gauge during the filling process.
Sensitivity to Ratios
In binary mixtures, a slight misreading of the initial partial pressure will skew the final percentage of both gases. The high-precision gauge is the only defense against creating an atmosphere that differs from the calculated theoretical values.
Making the Right Choice for Your Goal
To ensure your magnesium combustion data is scientifically valid, you must prioritize the fidelity of your gas delivery system.
- If your primary focus is Data Integrity: Ensure your pressure gauge is calibrated to at least 0.1% accuracy to minimize composition errors.
- If your primary focus is Environmental Range: Use the partial pressure method to systematically vary CO2 concentrations between 10% and 80% to observe trend lines in combustion behavior.
Precision in your pressure readings is not just a safety measure; it is the prerequisite for reproducible scientific discovery.
Summary Table:
| Feature | Specification/Method | Impact on Experiment |
|---|---|---|
| Mixing Technique | Partial Pressure Method | Enables precise binary gas ratios (CO2/N2) |
| Gauge Accuracy | 0.1% Precision Standard | Minimizes margin of error in gas composition |
| Concentration Range | 10% to 80% CO2 | Allows testing across diverse combustion scenarios |
| Key Function | Real-time Pressure Monitoring | Provides fundamental basis for data validity |
Elevate Your Combustion Research with KINTEK Precision
Precise gas composition is the foundation of reproducible scientific discovery. Backed by expert R&D and manufacturing, KINTEK offers high-performance equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable for your unique lab high-temp furnace needs.
Whether you are conducting magnesium combustion studies or advanced materials synthesis, our team provides the precision tools required to ensure your data integrity. Contact us today to discuss your custom furnace requirements and see how our expertise can optimize your experimental outcomes.
Visual Guide
References
- Ioan Barabulica, Ioan Mămăligă. Experimental Study on the Reaction of Magnesium in Carbon Dioxide and Nitrogen Atmosphere. DOI: 10.3390/chemengineering8020041
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
People Also Ask
- What is the primary function of a high-purity vacuum-sealed quartz tube in the Modified Bridgman technique? Key Role
- Why is a high-purity alumina (Al2O3) crucible required for the melting of nickel-based superalloys?
- Why is toluene used as a grinding aid in wet ball milling? Master Fine Metal Powder Synthesis with PCAs
- What role does a laboratory graphite box play during the selenization of CBTSe thin films? Key Synthesis Benefits
- What are the advantages of using high-purity quartz boats? Ensure Purity in Carbon Nanotube Synthesis
- What role do contact thermocouples play during the high-temperature annealing experiments of oriented silicon steel?
- What is the technical role of a magnetic stirring hot plate in synthesis? Optimize Cobalt Oxide Nanoparticle Quality
- What function do graphite chill plates or chill rings perform? Master Single-Crystal Blade Directional Solidification



















