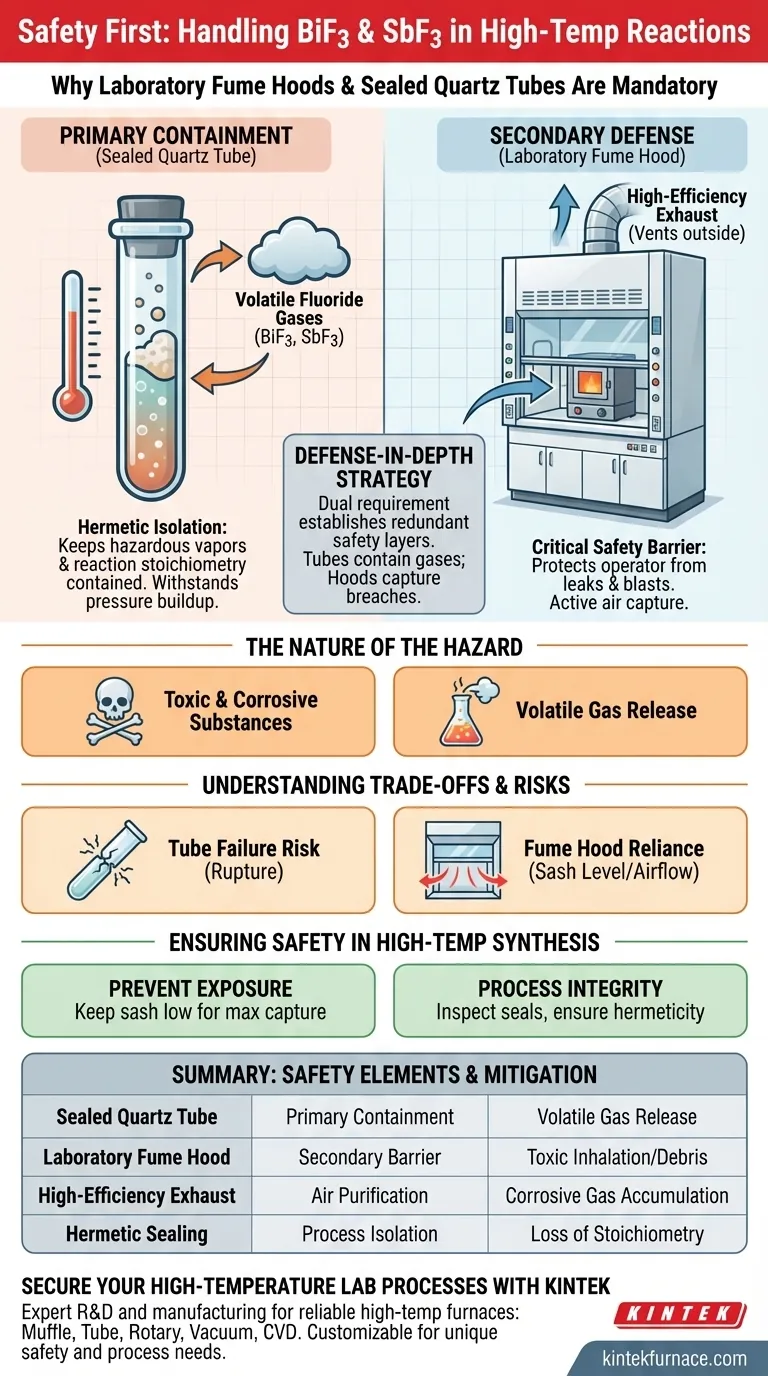
Laboratory fume hoods and sealed quartz tubes are mandatory because bismuth trifluoride (BiF3) and antimony trifluoride (SbF3) act as corrosive, toxic sources that liberate hazardous vapors when heated. The sealed tubes prevent the initial escape of reaction gases, while the fume hood serves as a critical secondary barrier to exhaust any leakage away from the operator.
The dual requirement of sealed quartz tubes and fume hoods establishes a "defense-in-depth" safety strategy: the tubes physically contain the volatile fluoride gases, while the exhaust system ensures that any breach does not result in personnel exposure.

The Nature of the Hazard
Toxicity and Corrosiveness
BiF3 and SbF3 are not benign reagents. They are inherently corrosive and toxic substances.
Handling them requires strict protocols to prevent direct contact or inhalation.
Volatile Gas Release
The primary danger arises during the high-temperature phase of the reaction.
When subjected to heat, these compounds release volatile fluoride gases. These vapors are highly mobile and hazardous if allowed to enter the laboratory atmosphere.
Primary Containment: Sealed Quartz Tubes
Hermetic Isolation
The quartz tube serves as the first line of defense. By using a hermetically sealed vessel, you isolate the chemical process completely from the external environment.
This containment is essential to keep the reaction stoichiometry intact and the hazardous byproducts inside the vessel.
Pressure Management
As the temperature rises and gases are released, internal pressure within the tube increases.
The sealed quartz construction is designed to withstand these conditions, effectively containing the reaction at the source.
Secondary Defense: Laboratory Fume Hoods
The Critical Safety Barrier
Physical containment can fail; glass can crack, and seals can break.
The laboratory fume hood acts as a necessary safety barrier, protecting the operator from potential blasts or leaks originating from the tube.
High-Efficiency Exhaust
Fume hoods rely on high-efficiency exhaust systems.
These systems actively pull air away from the user, capturing hazardous vapors and venting them out of the building to prevent the contamination of the laboratory air supply.
Understanding the Trade-offs
The Risk of Tube Failure
While sealed quartz is effective, it is also brittle.
If the internal pressure generated by the fluoride gases exceeds the tube's limits, the vessel can rupture, instantly releasing the toxic contents.
Reliance on Ventilation
A fume hood is only as effective as its user.
If the sash is raised too high or the exhaust flow is obstructed, the protective air barrier is compromised, allowing toxic vapors to escape into the breathing zone despite the ventilation system.
Ensuring Safety in High-Temperature Synthesis
To safely manage the risks of BiF3 and SbF3, apply the following protocols based on your operational focus:
- If your primary focus is preventing exposure: Keep the fume hood sash as low as possible to maximize the capture efficiency of the exhaust system.
- If your primary focus is process integrity: Ensure the quartz tubes are sealed hermetically and inspected for flaws to prevent containment failure under heat.
Redundancy is the key to safety; never rely on a single barrier when handling volatile toxic fluorides.
Summary Table:
| Safety Element | Primary Function | Specific Hazard Mitigated |
|---|---|---|
| Sealed Quartz Tube | Primary Containment | Volatile gas release and pressure buildup |
| Laboratory Fume Hood | Secondary Barrier | Toxic vapor inhalation and tube rupture debris |
| High-Efficiency Exhaust | Air Purification | Accumulation of corrosive fluoride gases |
| Hermetic Sealing | Process Isolation | Loss of stoichiometry and chemical leaks |
Secure Your High-Temperature Lab Processes with KINTEK
When handling volatile and corrosive materials like BiF3 and SbF3, equipment reliability is non-negotiable. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp furnaces, including Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your unique safety and process requirements.
Protect your personnel and ensure the integrity of your research with our precision-engineered solutions. Contact us today to discuss your custom furnace needs and see how KINTEK can enhance your lab's safety and efficiency.
Visual Guide
References
- Еvgeny V. Nazarchuk, Dmitri O. Charkin. A novel microporous uranyl silicate prepared by high temperature flux technique. DOI: 10.1515/zkri-2024-0121
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the function of molybdenum fixtures in high-temperature heat treatment? Ensure Perfect Diffusion Integrity
- How does a Mass Flow Controller (MFC) influence CrAlSiN coatings? Precision Ar/N2 Control for Hardness
- What are the properties and uses of ceramic tubes? Unlock High-Temp, Insulating Solutions
- How does the gas control system regulate the plasma nitriding process? Master Your N2/H2 Mixture for Superior Surfaces
- What are the advantages of using borosilicate glass for the upper atmosphere control chamber? Protect Your Vacuum Seals
- Why are magnesium oxide-stabilized zirconia crucibles used for melting alloys? High-Temp Stability up to 1900°C
- What are the electrical properties of alumina tubes? Discover Superior Insulation for Extreme Conditions
- What factors influence the lifespan of alumina ceramic furnace tubes? Maximize Durability and Performance



















