Magnesium oxide-stabilized zirconia crucibles are the definitive choice for high-temperature metallurgy because they solve the dual challenges of structural failure and chemical contamination. They are specifically engineered to withstand thermal shock and resist corrosion while processing alloys with melting points as high as 1900 degrees Celsius.
High-temperature alloys are notoriously difficult to process because they attack standard ceramic vessels and induce cracking during rapid heating. Magnesium oxide-stabilized zirconia mitigates these risks by combining exceptional thermal shock stability with chemical inertness, ensuring both vessel integrity and alloy purity.
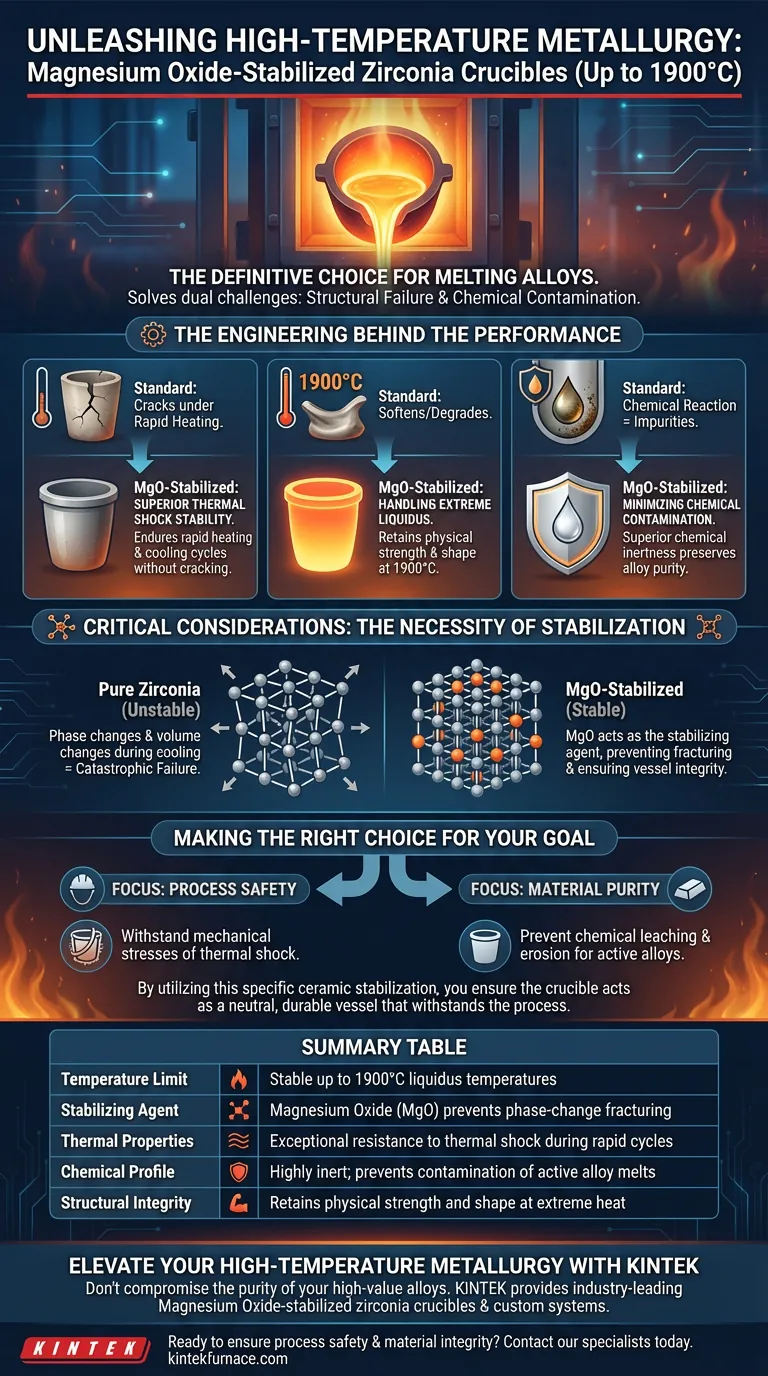
The Engineering Behind the Performance
Superior Thermal Shock Stability
The primary risk in high-temperature melting is the structural failure of the crucible due to rapid temperature changes.
Magnesium oxide stabilization modifies the crystal structure of the zirconia. This modification allows the crucible to endure the stress of heating and cooling cycles without cracking or shattering.
Handling Extreme Liquidus Temperatures
Standard refractory materials often soften or degrade before reaching the melting points of modern superalloys.
Magnesium oxide-stabilized zirconia retains its physical strength and shape at exceptionally high operating temperatures. This capability is essential for processing materials with liquidus temperatures up to 1900 degrees Celsius.
Minimizing Chemical Contamination
Molten alloys are highly active and tend to react aggressively with containment vessels, leading to impurities in the final product.
This material composition offers superior chemical inertness. It minimizes reactions between the crucible wall and active alloy melts, preserving the precise chemical composition of the metal being processed.
Critical Considerations
The Necessity of Stabilization
It is important to understand that pure zirconia alone is often unsuitable for these applications due to phase changes that occur during heating.
The addition of magnesium oxide is not merely an additive; it is the stabilizing agent that prevents catastrophic failure. Without this stabilization, the volume changes associated with cooling would likely cause the vessel to fracture, compromising the safety of the melt.
Making the Right Choice for Your Goal
When dealing with high-value alloys and extreme thermal environments, the margin for error is non-existent.
- If your primary focus is Process Safety: Rely on magnesium oxide-stabilized zirconia to withstand the mechanical stresses of thermal shock during rapid heating and cooling cycles.
- If your primary focus is Material Purity: Choose this composition to prevent chemical leaching and erosion when melting highly reactive or "active" alloys.
By utilizing this specific ceramic stabilization, you ensure the crucible acts as a neutral, durable vessel that withstands the process rather than becoming part of it.
Summary Table:
| Feature | Performance Benefit |
|---|---|
| Temperature Limit | Stable up to 1900°C liquidus temperatures |
| Stabilizing Agent | Magnesium Oxide (MgO) prevents phase-change fracturing |
| Thermal Properties | Exceptional resistance to thermal shock during rapid cycles |
| Chemical Profile | Highly inert; prevents contamination of active alloy melts |
| Structural Integrity | Retains physical strength and shape at extreme heat |
Elevate Your High-Temperature Metallurgy with KINTEK
Don't compromise the purity of your high-value alloys. KINTEK provides industry-leading Magnesium Oxide-stabilized zirconia crucibles designed to withstand the most demanding thermal environments.
Backed by expert R&D and precision manufacturing, we offer a full range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside customizable high-temp lab furnaces tailored to your unique research or production needs.
Ready to ensure process safety and material integrity?
Contact our specialists today to find your perfect solution.
Visual Guide
References
- Kilian Sandner, Uwe Glatzel. Investment casting of Cr–Si alloys with liquidus temperatures up to 1900 °C. DOI: 10.1007/s40962-024-01490-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why are high-purity alumina crucibles selected for lithium orthosilicate synthesis? Ensure Purity & Thermal Stability
- What factors should be considered when selecting an alumina ceramic furnace tube? Ensure Safety and Performance in High-Temp Processes
- What role does a water saturator play in the physical activation of carbon materials? Unlock High-Performance Porosity
- What characteristics are required for reaction vessels in PI-COFs synthesis? Ensure High-Pressure Safety and Purity
- What role does a high-purity alumina crucible play in melting tellurite glass? Ensure Optical Purity and Stability
- What is the temperature range for Laboratory Type Furnaces? Find Your Ideal Heat Solution
- Why is a high-precision mass flow controller (MFC) necessary in ferronickel alloy smelting? Ensure Metal Purity
- Why is a high-precision electronic balance critical in the formulation of geopolymer binders? Precision for Success



















