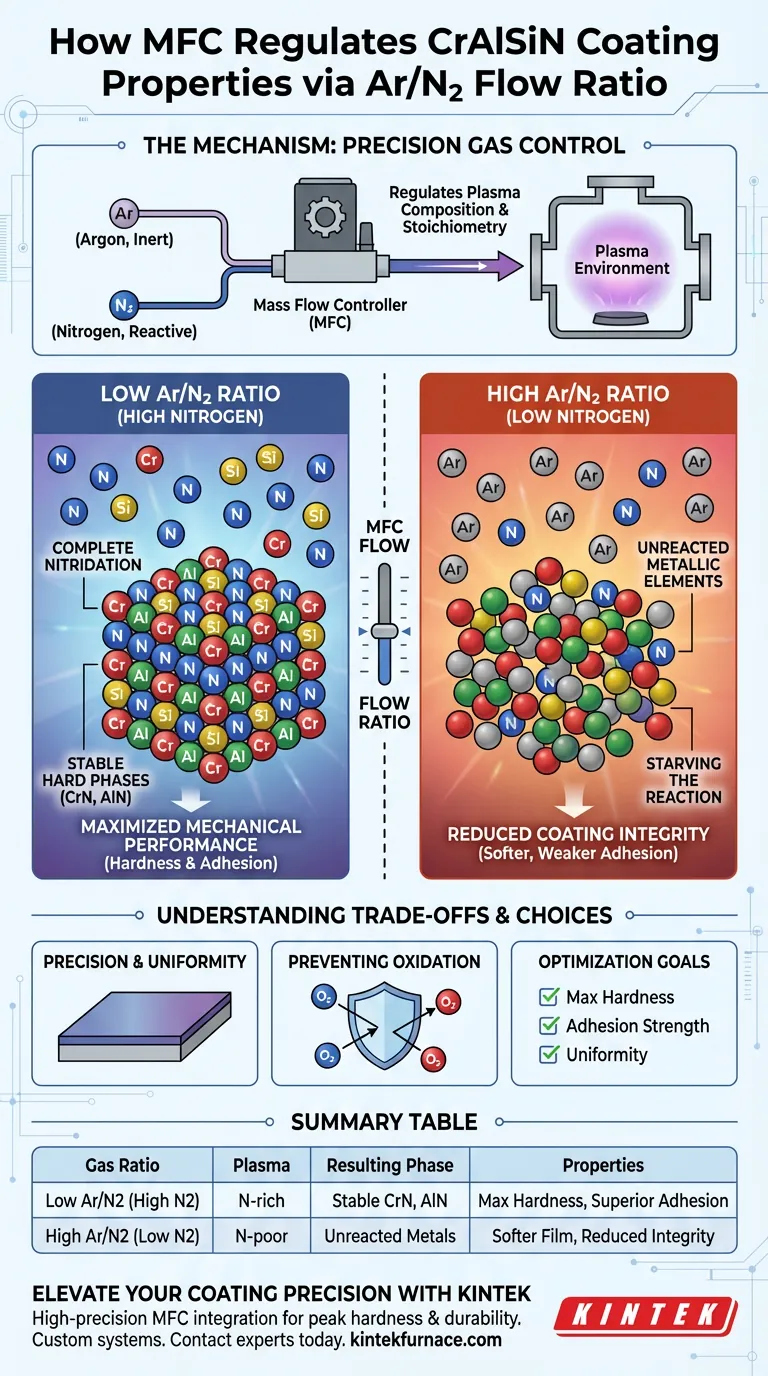
By strictly controlling the ratio of argon to nitrogen, a Mass Flow Controller (MFC) determines the fundamental chemical composition of the plasma during the deposition process. This regulation dictates whether metal atoms fully react to form hard ceramics or remain in a softer, metallic state, directly controlling the hardness and adhesion of CrAlSiN coatings.
Core Takeaway Precision gas control is the leverage point for coating quality. A lower Argon-to-Nitrogen ratio ensures a nitrogen-rich environment, driving the complete transformation of metals into stable, hard ceramic phases like CrN and AlN, whereas a lack of nitrogen compromises structural integrity.

The Mechanism of Control
Regulating Plasma Composition
The MFC acts as the gatekeeper for the reactive environment within the vacuum chamber.
By accurately metering the flow rates, the MFC sets the specific concentration of reactive gases (nitrogen) relative to inert gases (argon).
Determining Phase Stoichiometry
The ratio established by the MFC directly influences the stoichiometry of the final film.
It determines if there are enough nitrogen atoms present to bond with every sputtered metal atom (Chromium, Aluminum, Silicon).
Impact of a Low Ar/N2 Ratio (High Nitrogen)
Promoting Complete Nitridation
A lower Ar/N2 ratio indicates a higher concentration of nitrogen within the system.
This abundance promotes the complete nitridation of the metal atoms ejected from the target.
Formation of Stable Hard Phases
With sufficient nitrogen, the process favors the creation of stable, stoichiometric hard ceramic phases.
Specifically, this facilitates the crystallization of CrN (Chromium Nitride) and AlN (Aluminum Nitride) structures.
Maximizing Mechanical Performance
The presence of these fully reacted ceramic phases directly correlates to superior coating properties.
Users will observe significantly higher coating hardness and improved adhesion strength to the substrate.
Consequences of a High Ar/N2 Ratio (Low Nitrogen)
Starving the Reaction
A higher Ar/N2 ratio restricts the availability of reactive nitrogen in the plasma.
This creates a "nitrogen-poor" environment where the chemical reaction cannot sustain itself fully.
Unreacted Metallic Elements
When nitrogen is insufficient, metal atoms settle on the substrate without forming a bond.
This leads to the inclusion of unreacted metallic elements within the film matrix.
Reduced Coating Integrity
The presence of pure metal within a ceramic coating acts as a structural defect.
This results in a softer film with reduced overall performance and weaker adhesion.
Understanding the Trade-offs
The Necessity of Precision
While the primary goal is often high hardness, the MFC's role is also to maintain process consistency.
Fluctuations in flow—even minor ones—can lead to distinct layers within the coating where hardness varies, creating weak points.
Preventing Oxidation
Beyond the Ar/N2 ratio, the MFC must strictly regulate the carrier gas (Argon) to maintain positive pressure.
This effectively purges air impurities, preventing the material oxidation that can occur if the system pressure drops or fluctuates.
Making the Right Choice for Your Goal
To optimize your CrAlSiN coating process, you must tune the MFC to match your specific performance requirements.
- If your primary focus is Maximum Hardness: Prioritize a lower Ar/N2 ratio to ensure the formation of hard stoichiometric phases like CrN and AlN.
- If your primary focus is Adhesion Strength: Maintain high nitrogen flow to eliminate unreacted metallic inclusions that can weaken the interface between the coating and substrate.
- If your primary focus is Uniformity: Ensure your MFC is calibrated to prevent flow drift, as consistency in the gas ratio is required to maintain properties across the entire film thickness.
The MFC is not just a valve; it is a chemical switch that determines whether you deposit a high-performance ceramic or a compromised metallic film.
Summary Table:
| Gas Ratio Condition | Plasma Environment | Resulting Phase Composition | Mechanical Properties |
|---|---|---|---|
| Low Ar/N2 (High N2) | Nitrogen-rich | Stable CrN and AlN ceramic phases | Maximum hardness & superior adhesion |
| High Ar/N2 (Low N2) | Nitrogen-poor | Unreacted metallic inclusions | Softer film & reduced structural integrity |
| Inconsistent Flow | Fluctuating | Non-uniform layers & weak points | Poor durability & inconsistent performance |
Elevate Your Coating Precision with KINTEK
Don't let inconsistent gas flow compromise your material performance. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems, including advanced Mass Flow Control integration to ensure your CrAlSiN coatings achieve peak hardness and durability. Our systems are fully customizable to meet your unique laboratory or industrial needs.
Ready to optimize your deposition process? Contact our experts today to find the perfect high-temperature furnace solution for your application.
Visual Guide
References
- Cheng‐Hsun Hsu, Z. Chang. Improvement in Surface Hardness and Wear Resistance of ADI via Arc-Deposited CrAlSiN Multilayer Films. DOI: 10.3390/ma18092107
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Ultra Vacuum Electrode Feedthrough Connector Flange Power Lead for High Precision Applications
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
People Also Ask
- Why is the precise regulation of oxygen ratios via mass flow controllers critical for MCTV catalyst yield?
- Why is a mass flow controller essential in the tracer method? Precision Data for Pyrolysis Gas Flow
- What process challenges are addressed by vacuum filtration equipment during the construction of CsPbBr3@CA-SiO2 films?
- How does an infrared (IR) pyrometer improve thermal control? Direct Precision for MBE Growth and Annealing
- What function do the piping and butterfly valve components serve in a multi-kiln carbonization system? Maximize Control
- Why are high-purity alumina crucibles used for MAX phase sintering? Ensure Purity in High-Temperature Synthesis
- Why is a quartz boat required during APCVD for MoO2? Ensure High-Purity Single-Crystal Nanobelt Synthesis
- How does the sealed Alumina Tube structure benefit the design of a reference electrode? Boost Electrolysis Precision



















