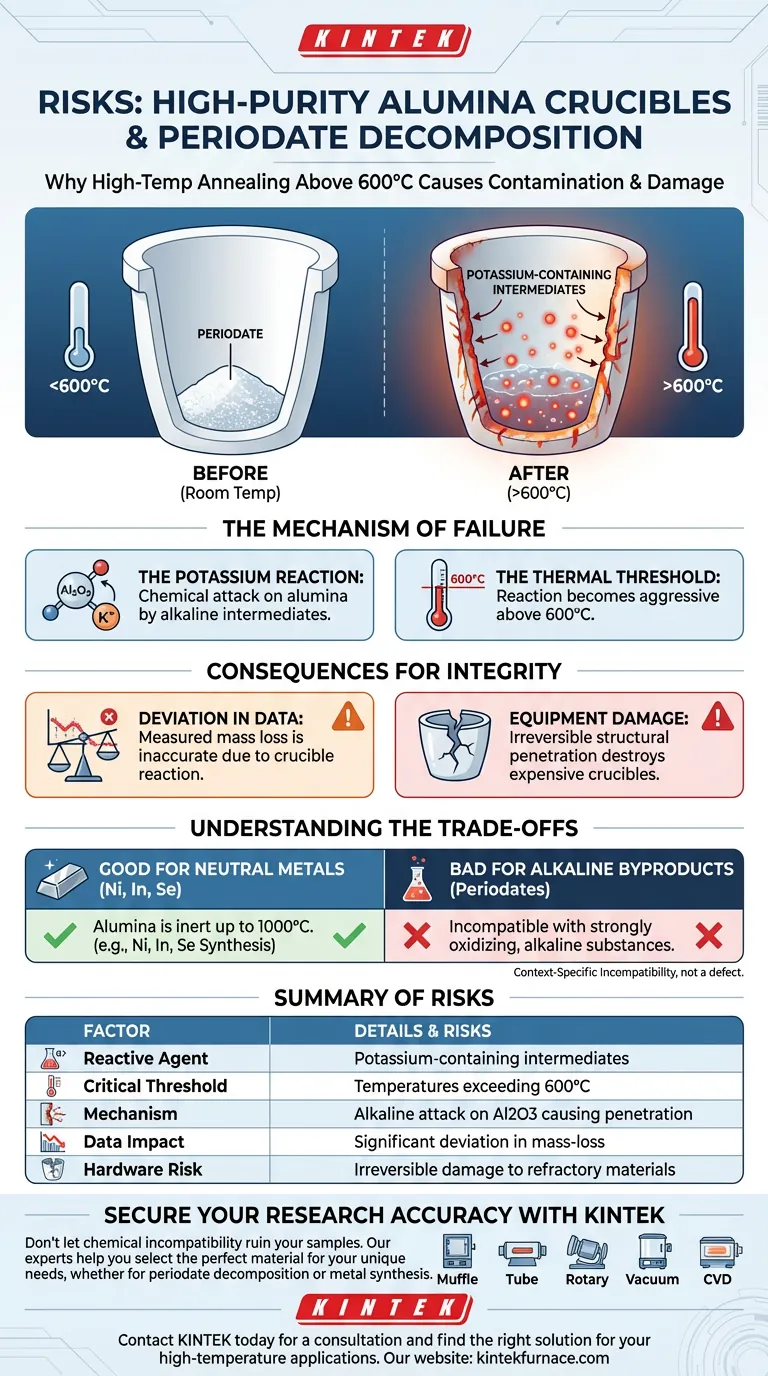
Using high-purity alumina crucibles for annealing periodate decomposition products poses a significant risk of chemical contamination and equipment damage. When temperatures exceed 600°C, potassium-containing intermediates generated during decomposition react aggressively with the alumina walls, leading to crucible penetration and highly inaccurate experimental data.
While high-purity alumina is widely regarded for its thermal resistance, it is not chemically inert to strongly alkaline substances. In the context of periodate decomposition, this incompatibility leads to a chemical reaction that compromises both the integrity of the containment vessel and the validity of your mass-loss measurements.
The Mechanism of Failure
The Potassium Reaction
The primary risk stems from the chemical nature of the decomposition products. As periodates break down, they generate potassium-containing intermediates.
These intermediates are not passive; they are chemically active and alkaline. They attack the aluminum oxide (Al2O3) structure, leading to a reaction between the sample and the vessel itself.
The Thermal Threshold
This reaction is temperature-dependent. The critical threshold for this failure mode is roughly 600°C.
Below this temperature, the risk may be manageable, but once the annealing process surpasses this point, the kinetic energy allows the potassium intermediates to physically penetrate the crucible walls.
Consequences for Experimental Integrity
Deviation from Theoretical Values
The most immediate scientific consequence is the corruption of your data. In gravimetric analysis or mass-loss studies, you rely on the crucible being a neutral container.
Because the sample is reacting with and penetrating the crucible, the measured mass loss will deviate significantly from theoretical expectations. You are no longer measuring just decomposition; you are measuring a complex side reaction.
Damage to Refractory Materials
Beyond the data, there is a physical cost. The penetration of the crucible walls causes irreversible structural damage.
High-purity alumina crucibles are expensive consumables. This reaction essentially destroys them after a single use, increasing the operational cost of the experiment significantly.
Understanding the Trade-offs
The "High-Purity" Trap
It is easy to assume that "high-purity" equates to universal chemical inertness, but this is a misconception.
For many applications, such as the synthesis of Nickel, Indium, or Selenium compounds, alumina is an excellent choice. It can withstand temperatures up to 1000°C without contaminating these specific melts.
Context-Specific Incompatibility
The failure here is not a defect in the alumina, but a mismatch in chemical compatibility.
Alumina performs exceptionally well with neutral metals and melts. However, it is vulnerable to strongly oxidizing and alkaline substances, such as the potassium byproducts of periodates. Using the wrong vessel for the specific chemistry of your sample is a common source of experimental error.
Making the Right Choice for Your Goal
To ensure the success of your high-temperature processes, evaluate your materials based on chemical compatibility, not just thermal ratings.
- If your primary focus is analyzing periodate decomposition: You must avoid alumina crucibles if heating above 600°C; the reaction with potassium intermediates will invalidate your mass-loss data.
- If your primary focus is synthesizing neutral metal compounds (e.g., Ni, In, Se): High-purity alumina remains a superior choice due to its proven inertness and stability during long thermal soaking periods at high temperatures.
Select your crucible material based on the specific chemical reactivity of your byproducts, not just the temperature of your furnace.
Summary Table:
| Factor | Details & Risks |
|---|---|
| Reactive Agent | Potassium-containing intermediates |
| Critical Threshold | Temperatures exceeding 600°C |
| Mechanism | Alkaline attack on Al2O3 causing structural penetration |
| Data Impact | Significant deviation in mass-loss measurements |
| Hardware Risk | Irreversible damage to expensive refractory materials |
Secure Your Research Accuracy with KINTEK
Don't let chemical incompatibility ruin your samples or damage your high-temp equipment. Backed by expert R&D and manufacturing, KINTEK offers a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces and specialized crucibles designed for your unique needs.
Whether you are working with periodate decomposition or synthesizing neutral metal compounds (Ni, In, Se), our technical team will help you select the perfect material to ensure experimental integrity.
Contact KINTEK today for a consultation and find the right solution for your high-temperature applications.
Visual Guide
References
- Two Polymorphs of the Magnetic <i>Catena</i> ‐Orthoperiodato‐Cuprate(II) K <sub>3</sub> [CuIO <sub>6</sub> ]·4H <sub>2</sub> O from Ultra‐Alkaline Media. DOI: 10.1002/zaac.202500092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the water-saving benefit of using a water circulating vacuum pump? Save Over 10 Tons of Water Daily
- How do 15x80mm technical openings and seals boost electric furnace efficiency? Maximize Thermal Performance Today
- What is the function of glass tubes in molten-core thermal drawing? Precision Shaping and Chemical Isolation
- Why are Y2O3 ceramic crucibles preferred over Al2O3 for Y-DD5 superalloys? Discover the Superior Inertness of Yttria
- What is the primary use of a crucible furnace? Ideal for Melting Non-Ferrous Metals Efficiently
- What is the function of a specifically designed annealing vessel in SVA? Enhance Your Film Crystallization Today
- What role do quartz tubes and vacuum sealing play in synthesis? Master High-Reactivity Compounds like U0.92Mn3Si2C
- What is the purpose of using a corundum crucible and graphite powder? Optimize Your High-Entropy Alloy Annealing



















