Water Cooled Copper Pots are the critical solution for melting titanium because the metal becomes highly reactive in its molten state, capable of destroying and reacting with almost all standard refractory materials. By utilizing forced water cooling, these pots freeze the outer layer of the titanium to create a self-protecting "skull." This ensures the molten liquid stays contained within a shell of its own solid material, rather than touching the copper crucible itself.
The core value of this technology is the elimination of contamination; by forcing a solidified alloy shell to form on the crucible wall, the molten titanium is chemically isolated from the container, ensuring absolute purity.

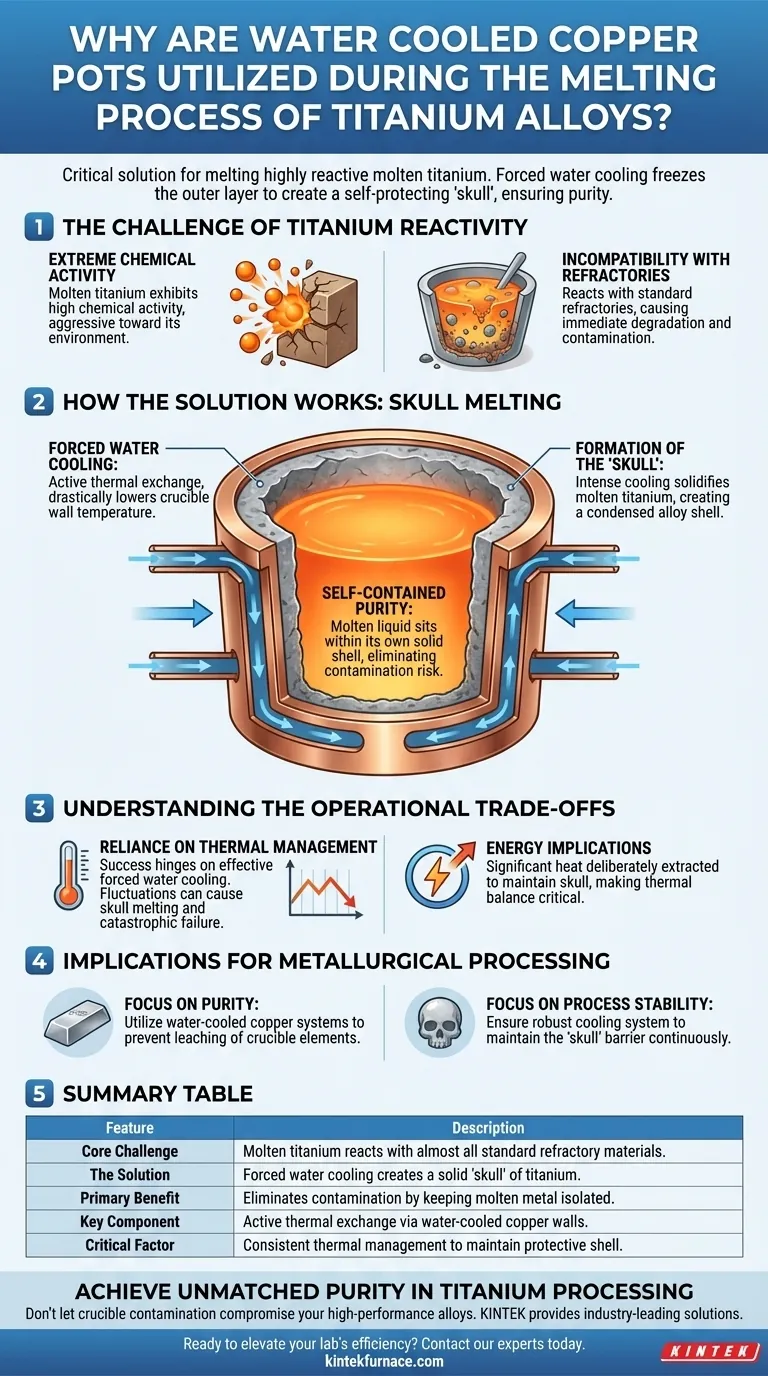
The Challenge of Titanium Reactivity
Extreme Chemical Activity
In its molten state, titanium is not chemically stable. It exhibits extremely high chemical activity, making it aggressive toward its environment.
Incompatibility with Refractories
Because of this high reactivity, titanium will react with almost all traditional refractory materials used in standard metallurgy. Using a standard ceramic or graphite crucible would result in immediate degradation of the vessel and contamination of the melt.
How the Solution Works: Skull Melting
Forced Water Cooling
The copper pot is not a passive container; it is an active thermal exchange system. It employs forced water cooling to drastically lower the temperature of the crucible's inner walls.
Formation of the "Skull"
This intense cooling causes the molten titanium to instantly solidify upon contact with the crucible wall. This creates a condensed alloy shell, technically referred to as a "skull."
Self-Contained Purity
Once the skull forms, the remaining liquid titanium sits inside this solid titanium shell. The molten metal only comes into contact with its own solid state, completely eliminating the risk of foreign material entering the alloy.
Understanding the Operational Trade-offs
Reliance on Thermal Management
The success of this process hinges entirely on the effectiveness of the forced water cooling. If the cooling mechanism fluctuates, the protective skull could melt, leading to catastrophic failure of the copper pot.
Energy Implications
This method inherently involves fighting the melting process at the crucible walls. A significant amount of heat is deliberately extracted to maintain the skull, making the thermal balance critical to operation.
Implications for Metallurgical Processing
To ensure high-integrity processing of reactive metals, consider these guiding principles:
- If your primary focus is Purity: You must utilize water-cooled copper systems to prevent the leaching of crucible elements into the titanium alloy.
- If your primary focus is Process Stability: You must ensure the forced water cooling system is robust enough to maintain the "skull" barrier continuously during the melt.
By leveraging the physics of the material against itself, water-cooled copper pots provide the only reliable method for melting reactive alloys without compromising their chemical composition.
Summary Table:
| Feature | Description |
|---|---|
| Core Challenge | Molten titanium reacts with almost all standard refractory materials. |
| The Solution | Forced water cooling creates a solid "skull" of titanium. |
| Primary Benefit | Eliminates contamination by keeping molten metal isolated from the crucible. |
| Key Component | Active thermal exchange via water-cooled copper walls. |
| Critical Factor | Consistent thermal management to maintain the protective alloy shell. |
Achieve Unmatched Purity in Titanium Processing
Don’t let crucible contamination compromise your high-performance alloys. KINTEK provides industry-leading high-temperature solutions tailored for reactive metals. Backed by expert R&D and manufacturing, we offer customizable Vacuum, CVD, and specialized melting systems designed to meet your unique metallurgical needs.
Ready to elevate your lab's efficiency and material integrity? Contact our experts today to discover the perfect thermal system for your applications.
Visual Guide
References
- Ahmed H. Awad, Shimaa El‐Hadad. Studying the Behavior of Cast and Thermally Treated α + β -Titanium Alloys Using the Abbott Firestone Technique. DOI: 10.1007/s40962-024-01528-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Induction Melting Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a vacuum arc melting furnace play in Ti-6Al-7Nb-xTa alloys? Precision Melting & Purity
- What are the technical advantages of using an induction furnace for lithium battery recycling over resistance heating?
- What is the function of a high-purity argon environment? Ensure Precision in Cu-Zn-Al-Sn Alloy Melting
- Why are induction furnaces suitable for investment casting? Precision Melting for Complex Casts
- What are the advantages of using PLCs in induction furnaces? Boost Efficiency and Quality with Automation
- What environmental benefits do induction melting furnaces provide? Reduce Emissions & Boost Efficiency
- Does induction heating work on graphite? Unlock Rapid, High-Temperature Processing
- What industries commonly use vacuum casting and for what applications? Discover Versatile Solutions for Prototyping and High-Performance Parts



















