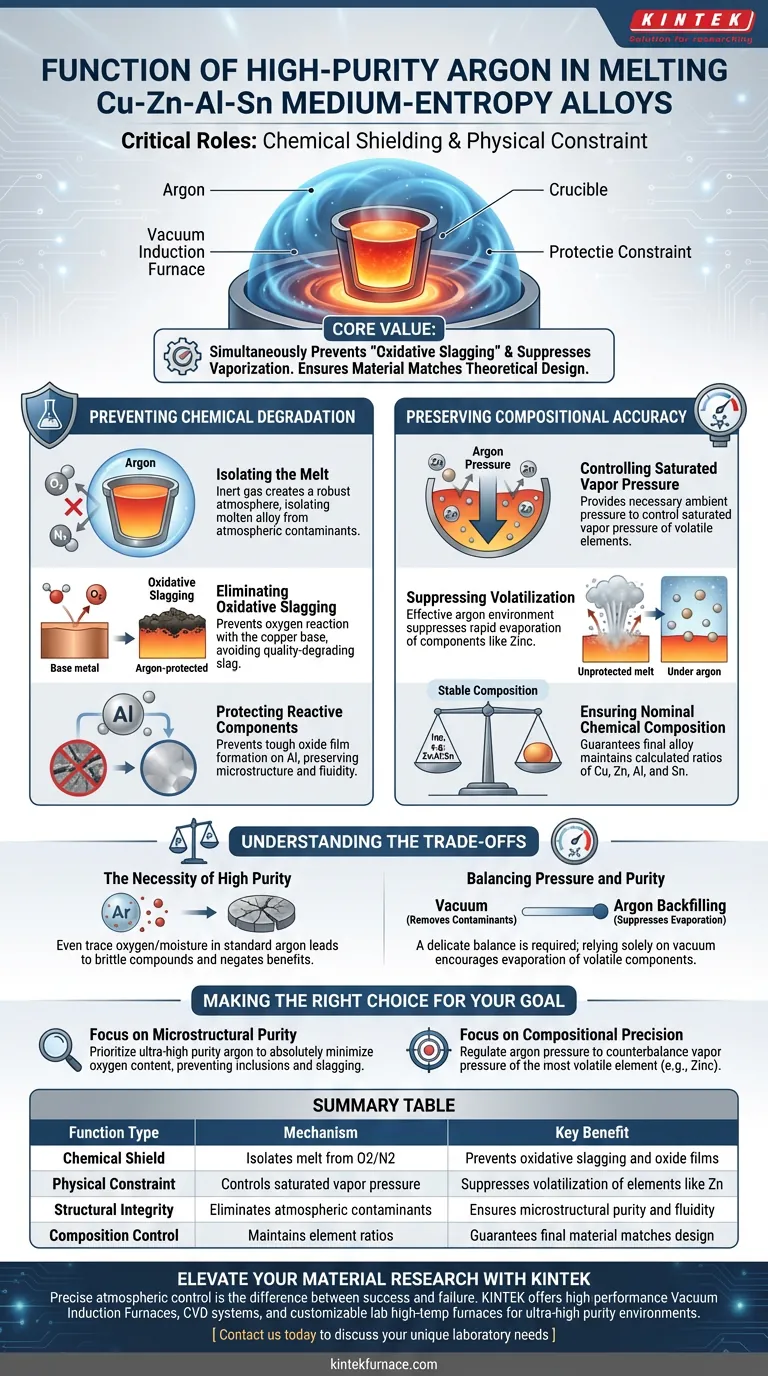
In the fabrication of Cu-Zn-Al-Sn medium-entropy alloys, high-purity argon serves two critical functions: it acts as a chemical shield against oxidation and a physical constraint against element evaporation. By establishing an inert environment within the vacuum induction furnace, argon ensures the final alloy retains both its intended structural purity and its precise chemical formulation.
The core value of a high-purity argon environment is its ability to simultaneously prevent "oxidative slagging" of the copper base and suppress the vaporization of volatile alloy components, ensuring the actual material matches the theoretical design.

Preventing Chemical Degradation
Isolating the Melt
The primary role of high-purity argon is to create a robust protective atmosphere. This inert gas effectively isolates the molten alloy from atmospheric contaminants, specifically oxygen and nitrogen.
Eliminating Oxidative Slagging
Without this isolation, the copper-based alloy is susceptible to "oxidative slagging." This process occurs when oxygen reacts with the melt, creating slag that degrades the material's quality.
Protecting Reactive Components
While the copper base requires protection, the aluminum (Al) component is particularly sensitive. Argon prevents the formation of tough oxide films that can compromise the microstructure and fluidity of the alloy.
Preserving Compositional Accuracy
Controlling Saturated Vapor Pressure
Beyond chemical protection, argon plays a vital physical role regarding the alloy's volatile components (such as Zinc). The gas provides the necessary ambient pressure to control the saturated vapor pressure of these elements.
Suppressing Volatilization
High-temperature melting can cause volatile elements to evaporate rapidly if uncontrolled. The argon environment effectively suppresses this volatilization, keeping these elements within the melt rather than allowing them to escape into the furnace chamber.
Ensuring Nominal Chemical Composition
By managing vapor pressure, the argon environment guarantees that the final alloy maintains its "nominal chemical composition." This ensures that the ratios of Cu, Zn, Al, and Sn remain exactly as calculated in the alloy design.
Understanding the Trade-offs
The Necessity of High Purity
Standard industrial argon is often insufficient for medium-entropy alloys. Even trace amounts of oxygen or moisture in the gas supply can lead to the formation of brittle compounds or surface oxides, negating the benefits of the protective atmosphere.
Balancing Pressure and Purity
There is a delicate balance between vacuum levels and argon backfilling. While a vacuum removes initial contaminants, relying solely on a vacuum would encourage the rapid evaporation of volatile components like Zinc; therefore, the introduction of argon is not optional but a requirement for compositional stability.
Making the Right Choice for Your Goal
To optimize your melting process for Cu-Zn-Al-Sn alloys, consider the following specific objectives:
- If your primary focus is Microstructural Purity: Prioritize the purity grade of your argon source to absolutely minimize oxygen content, preventing oxide inclusions and slagging.
- If your primary focus is Compositional Precision: Focus on regulating the argon pressure inside the furnace to specifically counterbalance the vapor pressure of the most volatile element (typically Zinc) in your mix.
Success in melting medium-entropy alloys lies in treating the atmosphere as an active processing tool, not just a passive shield.
Summary Table:
| Function Type | Mechanism | Key Benefit |
|---|---|---|
| Chemical Shield | Isolates melt from oxygen/nitrogen | Prevents oxidative slagging and oxide film formation |
| Physical Constraint | Controls saturated vapor pressure | Suppresses volatilization of elements like Zinc (Zn) |
| Structural Integrity | Eliminates atmospheric contaminants | Ensures microstructural purity and alloy fluidity |
| Composition Control | Maintains element ratios | Guarantees final material matches nominal chemical design |
Elevate Your Material Research with KINTEK
Precise atmospheric control is the difference between a successful melt and a failed alloy. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum Induction Furnaces, CVD systems, and customizable lab high-temp furnaces designed to maintain the ultra-high purity environments your research demands.
Whether you are working with complex medium-entropy alloys or sensitive reactive metals, our systems provide the pressure regulation and gas purity required for flawless results. Contact us today to discuss your unique laboratory needs and see how our tailored thermal solutions can enhance your manufacturing precision.
Visual Guide
References
- Spyridon Chaskis, Spyros Papaefthymiou. Compositional Design and Thermal Processing of a Novel Lead-Free Cu–Zn–Al–Sn Medium Entropy Brass Alloy. DOI: 10.3390/met14060620
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is electromagnetic induction used for in industrial applications? Achieve Fast, Precise Metal Heating
- What is the purpose of capacitors in an induction heater circuit? Amplify Heating Power and Efficiency
- Why is multiple remelting required for TNZTSF alloys? Achieve Total Homogeneity with Refractory Elements
- What are the advantages of using a vacuum casting furnace? Achieve Purity and Precision in Metal Processing
- How is the semi-levitation effect generated in induction cold crucible melting? Unlock Ultra-Pure Alloy Synthesis
- What are the experimental advantages of using a vacuum induction furnace for cast iron desulfurization research?
- What role does a crucible lid play during the vacuum induction smelting of AlV55 alloys? Boost Purity & Yield
- What is a Vacuum Induction Melting Furnace (VIM) and what processes does it perform? Achieve Ultimate Metal Purity and Precision



















