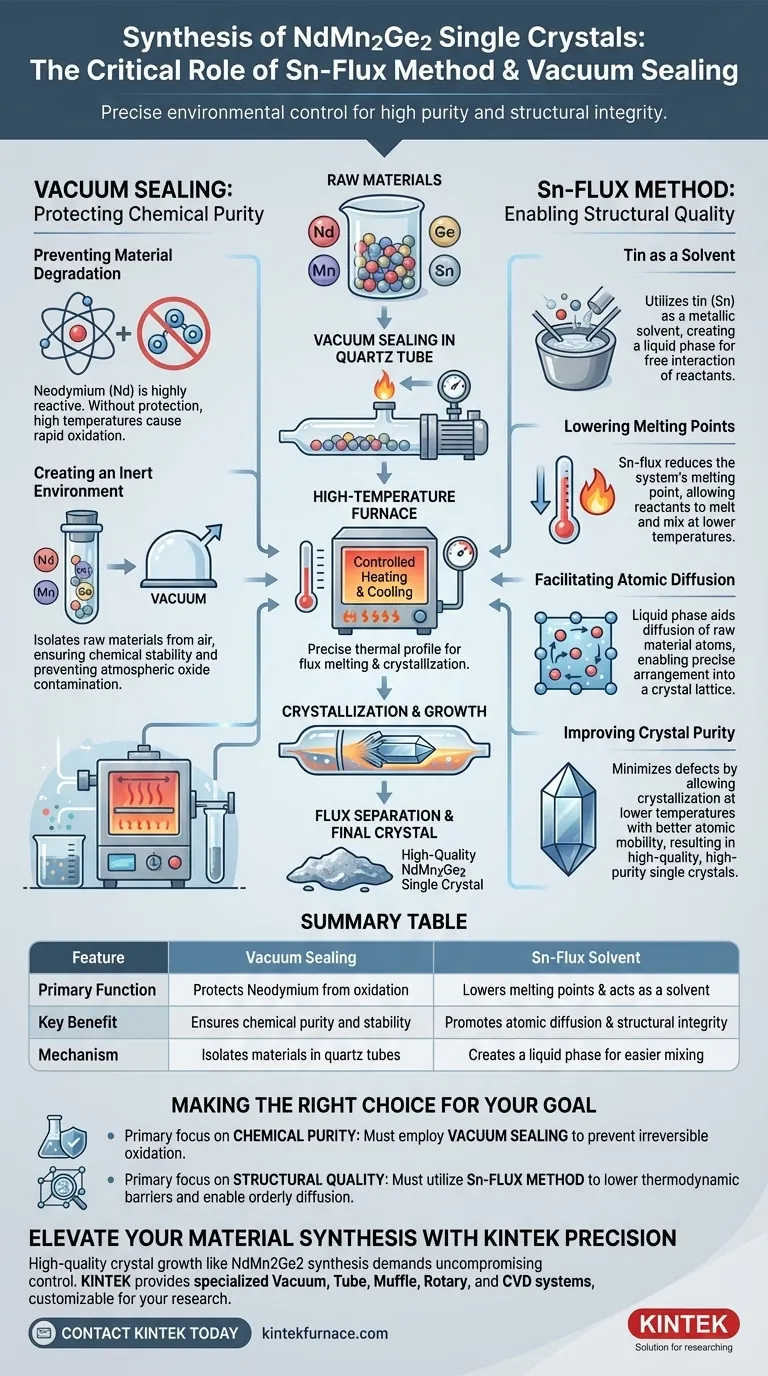
The synthesis of high-quality NdMn2Ge2 single crystals requires precise environmental control to ensure purity and structural integrity. Vacuum sealing is strictly necessary to prevent the oxidation of the highly reactive Neodymium (Nd) component. Meanwhile, the Sn-flux method is employed to lower the operating temperature and create a liquid environment that promotes the growth of high-purity, defect-free crystals.
Creating NdMn2Ge2 crystals is a balance of chemical protection and thermodynamic control. Vacuum sealing protects reactive rare earth elements from the atmosphere, while the tin (Sn) flux acts as a solvent to facilitate atomic diffusion and crystallization at manageable temperatures.

The Critical Role of Vacuum Sealing
Preventing Material degradation
Neodymium (Nd) is a rare earth element that is highly reactive with oxygen. Without protection, the high temperatures required for synthesis would cause rapid oxidation.
Creating an Inert Environment
Vacuum sealing the raw materials in quartz tubes isolates them completely from the air. This ensures that the chemical composition remains stable and the final crystal is not contaminated by atmospheric oxides.
The Mechanics of the Sn-Flux Method
Tin as a Solvent
The Sn-flux method utilizes tin (Sn) to act as a metallic solvent for the raw materials. This creates a liquid phase environment where the reactants can interact more freely than they would in a solid state.
Lowering Melting Points
A primary advantage of this method is the reduction of the system's melting point. The presence of the tin flux allows the reactants to melt and mix at lower temperatures than would be required for direct melting of the individual components.
Facilitating Atomic Diffusion
The liquid phase provided by the molten tin is critical for crystal quality. It allows for easier diffusion of raw material atoms, enabling them to arrange themselves into a distinct crystal lattice with greater precision.
Improving Crystal Purity
By allowing crystallization to occur at lower temperatures with better atomic mobility, the Sn-flux method minimizes defects. This process is instrumental in producing high-quality and high-purity single crystals.
Understanding the Trade-offs
Complexity vs. Quality
While direct synthesis methods might be faster, they often fail to produce single crystals of sufficient quality for this specific material. The Sn-flux and vacuum sealing methods add processing steps but are necessary investments to avoid defects and impurities.
Flux Separation
Using a flux introduces an additional material (tin) into the process. The success of this method relies on the flux aiding the reaction without becoming a permanent, unwanted contaminant in the final crystal structure.
Making the Right Choice for Your Goal
To ensure the successful synthesis of NdMn2Ge2, you must prioritize the specific function of each technique:
- If your primary focus is Chemical Purity: You must employ vacuum sealing to prevent the irreversible oxidation of Neodymium.
- If your primary focus is Structural Quality: You must utilize the Sn-flux method to lower thermodynamic barriers and enable the orderly diffusion of atoms into a single crystal lattice.
By combining an isolated vacuum environment with a flux-assisted growth process, you ensure the production of single crystals that are both chemically pure and structurally sound.
Summary Table:
| Feature | Method: Vacuum Sealing | Method: Sn-Flux Solvent |
|---|---|---|
| Primary Function | Protects Neodymium from oxidation | Lowers melting points & acts as a solvent |
| Key Benefit | Ensures chemical purity and stability | Promotes atomic diffusion & structural integrity |
| Mechanism | Isolates materials in quartz tubes | Creates a liquid phase for easier mixing |
| Result | Oxide-free raw materials | High-quality, defect-free single crystals |
Elevate Your Material Synthesis with KINTEK Precision
High-quality crystal growth like NdMn2Ge2 synthesis demands uncompromising thermal and atmospheric control. KINTEK provides the specialized equipment needed to master these complex processes. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Vacuum, Tube, Muffle, Rotary, and CVD systems, all fully customizable to meet your specific research or production requirements.
Whether you are working with reactive rare earth elements or advanced flux methods, our high-temperature furnaces provide the stability and vacuum integrity your lab needs. Contact KINTEK today to discuss your custom furnace solution and ensure your next synthesis is a success.
Visual Guide
References
- Samuel K. Treves, Valerio Scagnoli. Investigating skyrmion stability and core polarity reversal in NdMn2Ge2. DOI: 10.1038/s41598-024-82114-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How do vacuum furnaces work? Unlock Clean, High-Purity Heat Treatment
- What does the vacuum system of a vacuum furnace consist of? Essential Components for Clean Heat Processing
- How does a vacuum impregnation device facilitate PCMs into biomimetic composites? Boost Filling Rates to 96%
- Why is a cyclic heat treatment furnace required for the tempering or annealing of TiNi alloys after cold rolling?
- Why is a vacuum distillation apparatus necessary in the Kroll process? Achieving Purity in Zirconium Sponge Production
- What are the key features of a vacuum furnace? Achieve Purity and Precision in Material Processing
- Why is a laboratory vacuum drying oven necessary for SPC-Fe electrodes? Ensure Electrochemical Viability
- What role does a vacuum annealing furnace play in Bi4I4 single crystals? Master Precise Fermi Level Engineering



















