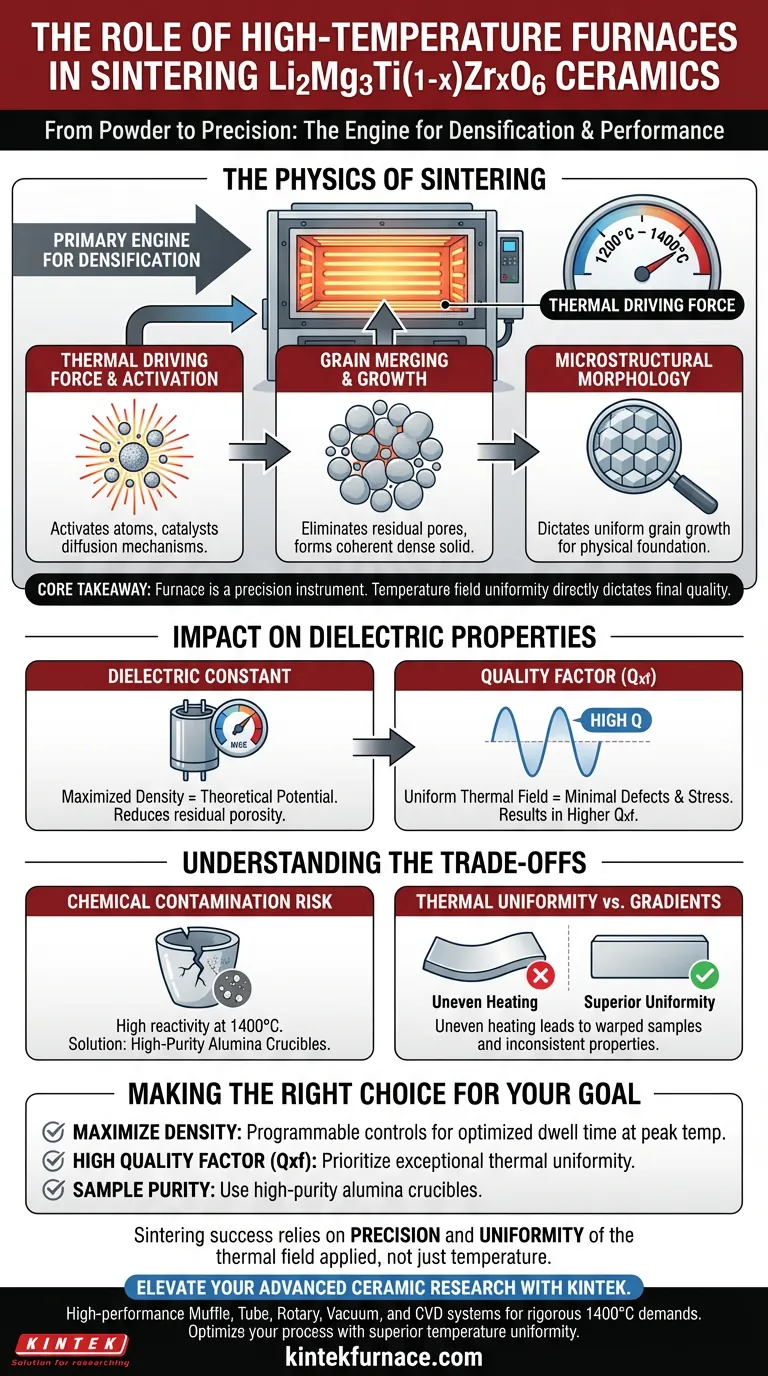
The high-temperature experimental furnace serves as the primary engine for densification in the production of Li2Mg3Ti(1-x)ZrxO6 ceramics. It provides the essential thermal driving force, specifically between 1200°C and 1400°C, to trigger diffusion mechanisms that transform the porous green body into a solid, dense material.
Core Takeaway The furnace is not merely a heat source; it is a precision instrument where temperature field uniformity directly dictates the ceramic's final quality. The accuracy of the heating profile determines the material's microstructure, which in turn defines critical performance metrics like the dielectric constant and the quality factor (Qxf).
The Physics of Sintering
The Thermal Driving Force
To achieve a solid ceramic state, the material requires substantial energy to overcome kinetic barriers. The furnace provides a sustained environment of 1200°C to 1400°C.
This high thermal energy activates the atoms within the ceramic green body. It acts as the catalyst for the diffusion mechanisms required for solid-state reactions.
Grain Merging and Growth
As the temperature rises, the individual grains within the ceramic powder begin to merge. This process is driven by the reduction of surface energy.
The furnace facilitates the elimination of residual pores located between these grains. The result is a transition from a loosely packed powder structure to a coherent, dense solid.
Microstructural Morphology
The specific thermal profile applied by the furnace dictates how the grains grow and arrange themselves.
Precise control ensures the microstructure develops uniformly. This morphology is the physical foundation for the ceramic's mechanical and electrical properties.
Impact on Dielectric Properties
Defining the Dielectric Constant
The relationship between the sintering process and the material's electrical capabilities is direct.
The furnace's ability to maximize density ensures the dielectric constant reaches its theoretical potential. Residual porosity would severely degrade this value.
Optimizing the Quality Factor (Qxf)
For microwave dielectric ceramics like Li2Mg3Ti(1-x)ZrxO6, the Quality Factor (Qxf) is a paramount performance metric.
The furnace's stability ensures the crystalline structure forms with minimal defects. A uniform thermal field minimizes internal stress and lattice distortions, resulting in a higher Qxf.
Understanding the Trade-offs
The Risk of Chemical Contamination
While the furnace provides the heat, the interaction between the sample and the furnace environment can be detrimental. At 1400°C, ceramics are highly reactive.
Direct contact with furnace linings can introduce impurities. To mitigate this, high-purity alumina crucibles are often required to chemically isolate the sample and maintain purity.
Thermal Uniformity vs. Gradients
A common pitfall in experimental sintering is uneven heating.
If the furnace lacks superior temperature field uniformity, the ceramic will densify unevenly. This leads to warped samples and inconsistent dielectric properties across the material.
Making the Right Choice for Your Goal
To maximize the performance of Li2Mg3Ti(1-x)ZrxO6 ceramics, consider the following specific adjustments:
- If your primary focus is maximizing Density: Ensure the furnace is capable of programmable heating controls to optimize the dwell time at the peak temperature (1200°C–1400°C) to fully eliminate pores.
- If your primary focus is High Quality Factor (Qxf): Prioritize a furnace with exceptional thermal uniformity to prevent microstructural gradients that increase dielectric loss.
- If your primary focus is Sample Purity: Utilize high-purity alumina crucibles within the furnace to prevent chemical reactions with the furnace lining.
The success of your sintering process relies less on maximum temperature and more on the precision and uniformity of the thermal field applied.
Summary Table:
| Process Factor | Impact on Ceramic Properties | Recommended Range/Solution |
|---|---|---|
| Sintering Temp | Triggers diffusion and densification | 1200°C – 1400°C |
| Thermal Uniformity | Determines Qxf and microstructure consistency | Precision programmable control |
| Pore Elimination | Maximizes dielectric constant potential | Optimized dwell times |
| Chemical Purity | Prevents degradation of material properties | High-purity alumina crucibles |
Elevate Your Advanced Ceramic Research with KINTEK
Precision in the thermal field is the difference between a flawed sample and a record-breaking Quality Factor (Qxf). Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous 1400°C demands of microwave dielectric ceramic sintering.
Whether you need superior temperature uniformity for grain growth or customizable heating profiles for densification, our lab high-temperature furnaces are engineered for your unique research needs.
Ready to optimize your sintering process? Contact our experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Weihua Li, Haiguang Zhao. Highly bright solid-state carbon dots for efficient anticounterfeiting. DOI: 10.1039/d3ra07235e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the common applications of benchtop furnaces? Unlock Precision in Materials Science and More
- How does heat treatment in a muffle furnace affect CuFe2O4–SnO2-rGO? Optimize Heterojunction Composite Performance
- What is the role of a Muffle Furnace in the thermal modification of wood? Optimize Daniellia oliveri Durability
- What are the main advantages of crucible furnaces? Achieve Precision & Flexibility in Small-Batch Melting
- How does high-temperature calcination in a muffle furnace transform precipitates? Expert Insights into Oxide Synthesis
- Why is a high-temperature muffle furnace required for the roasting of activated fly ash? Unlock Efficient Phase Changes
- How does an adjustable thermal gradient benefit sensitive samples in muffle furnaces? Prevent Thermal Shock and Ensure Precision
- How is a muffle furnace applied in the food industry? Essential for Ash and Moisture Analysis



















