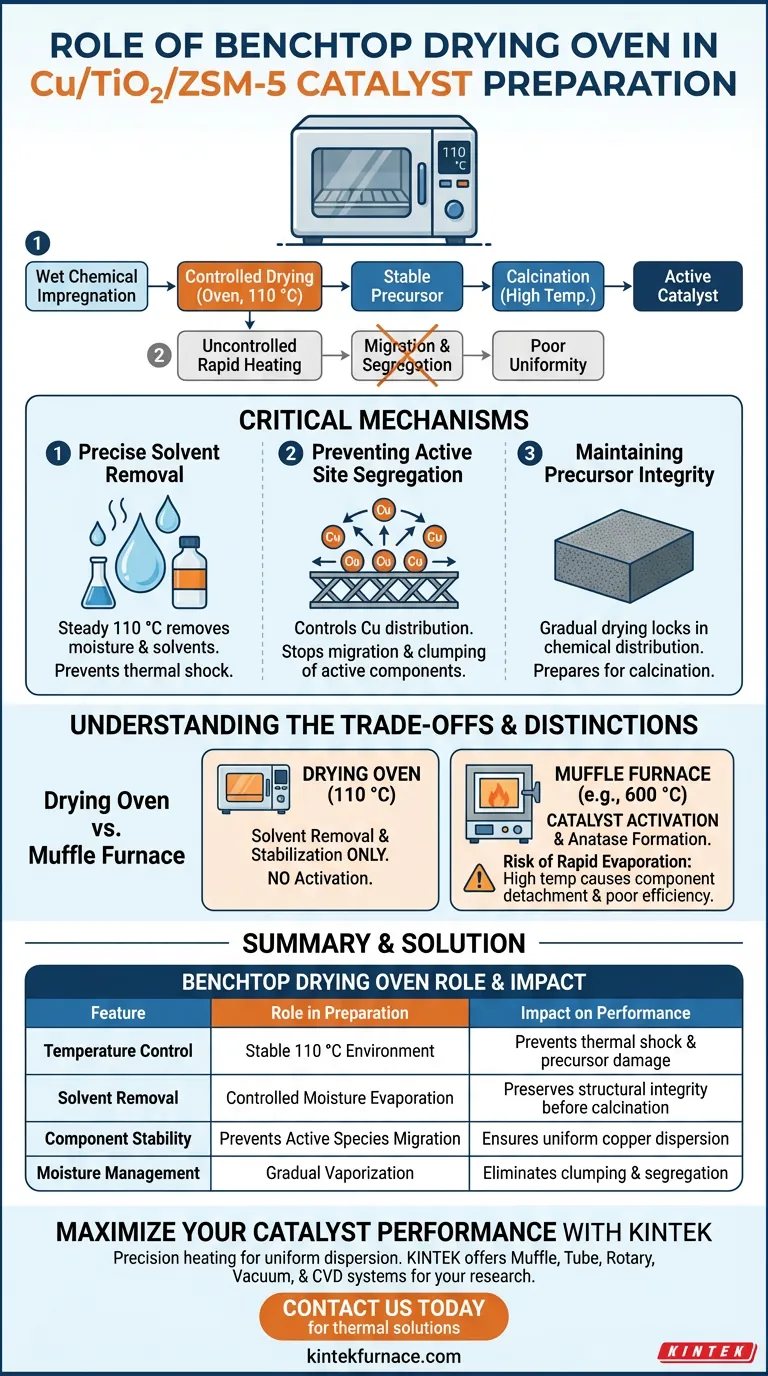
The primary role of a benchtop drying oven in preparing Cu/TiO2/ZSM-5 catalysts is to provide a controlled, constant temperature environment, typically at 110 °C, to remove solvents and moisture following wet chemical impregnation. By regulating the rate of evaporation, this step secures the structural integrity of the precursor before high-temperature activation.
Core Takeaway While the mechanical function of the oven is simple solvent removal, its distinct chemical purpose is to "freeze" the distribution of active components in place. A controlled drying process is the only way to prevent the migration or severe segregation of copper species, ensuring uniform dispersion on the catalyst surface.

The Critical Mechanisms of Drying
Precise Solvent Removal
The benchtop drying oven (specifically an electric thermostatic blast drying oven) operates at a steady 110 °C.
This temperature is selected to effectively drive off moisture and solvents used during the impregnation phase without subjecting the material to thermal shock.
Preventing Active Site Segregation
The most vital function of this equipment is controlling the distribution of the copper (Cu) species.
If moisture evaporates too rapidly or unevenly, active components can migrate across the support surface.
This migration leads to severe segregation, where the copper clumps together rather than remaining evenly dispersed.
Maintaining Precursor Integrity
By ensuring a gradual drying process, the oven maintains the intended chemical distribution on the precursor surface.
This prepares the material for subsequent steps, ensuring that the copper species are locked into their optimal positions relative to the TiO2 and ZSM-5 support.
Understanding the Trade-offs
The Risk of Rapid Evaporation
Using a higher temperature device or uncontrolled heating to speed up this process is a common error.
Rapid vaporization can cause the active components to detach or aggregate, leading to a catalyst with poor uniformity and reduced efficiency.
Drying vs. Calcination
It is critical to distinguish the drying oven from the high-temperature muffle furnace.
The drying oven (110 °C) is strictly for solvent removal and component stabilization.
It does not activate the catalyst or convert the titanium precursors into the anatase TiO2 phase; that requires calcination at significantly higher temperatures (e.g., 600 °C) in a different furnace.
Making the Right Choice for Your Goal
To maximize the performance of your Cu/TiO2/ZSM-5 catalyst, apply the drying stage with specific intent:
- If your primary focus is Maximizing Dispersion: Ensure the oven temperature is strictly regulated at 110 °C to prevent the migration of copper species during solvent evaporation.
- If your primary focus is Structural Integrity: Allow sufficient time for thorough drying to prevent violent vaporization of residual moisture during the subsequent high-temperature calcination.
Controlled drying is not merely a preparation step; it is the foundation of catalyst uniformity.
Summary Table:
| Feature | Role in Catalyst Preparation | Impact on Performance |
|---|---|---|
| Temperature Control | Stable 110 °C environment | Prevents thermal shock and precursor damage |
| Solvent Removal | Controlled moisture evaporation | Preserves structural integrity before calcination |
| Component Stability | Prevents active species migration | Ensures uniform copper dispersion across the support |
| Moisture Management | Gradual vaporization | Eliminates clumping and severe active site segregation |
Maximize Your Catalyst Performance with KINTEK
Precision is the foundation of high-performance catalyst synthesis. At KINTEK, we understand that uniform dispersion of active sites starts with controlled, reliable heating.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique research needs. Whether you are drying precursors or performing high-temperature calcination, our systems ensure the thermal stability required for superior material science results.
Ready to elevate your lab’s efficiency? Contact us today to discuss your specific catalyst preparation requirements and find the perfect thermal solution for your research.
Visual Guide
References
- Wibawa Hendra Saputera, Dwiwahju Sasongko. Understanding the Role of Copper Oxidation State on a TiO<sub>2</sub>/ZSM‐5 Catalyst for Photocatalytic CO<sub>2</sub> Reduction to Methanol. DOI: 10.1002/admi.202500010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the mechanism by which a reducing atmosphere improves Mn-Zn ferrite performance? Unlocking Magnetic Excellence
- How does a programmable high-temperature annealing furnace improve AZO thin films? Master Your Atmosphere Control
- For which materials is the experimental box type atmosphere furnace suitable? Ideal for Metals, Ceramics, and Advanced Materials
- What is a vacuum atmosphere furnace? Master High-Purity Heat Treatment for Superior Materials
- What are the main application fields of atmosphere furnaces? Essential for Metal, Electronics, and R&D
- What are the considerations for air atmosphere and cooling in Inconel 625 heat treatment? Optimize 3D Part Stability
- How does a vacuum or controlled atmosphere furnace facilitate sessile-drop experiments? Optimize Alloy Wetting Analysis
- What are the features of continuous annealing furnaces? Boost High-Volume Production Efficiency



















