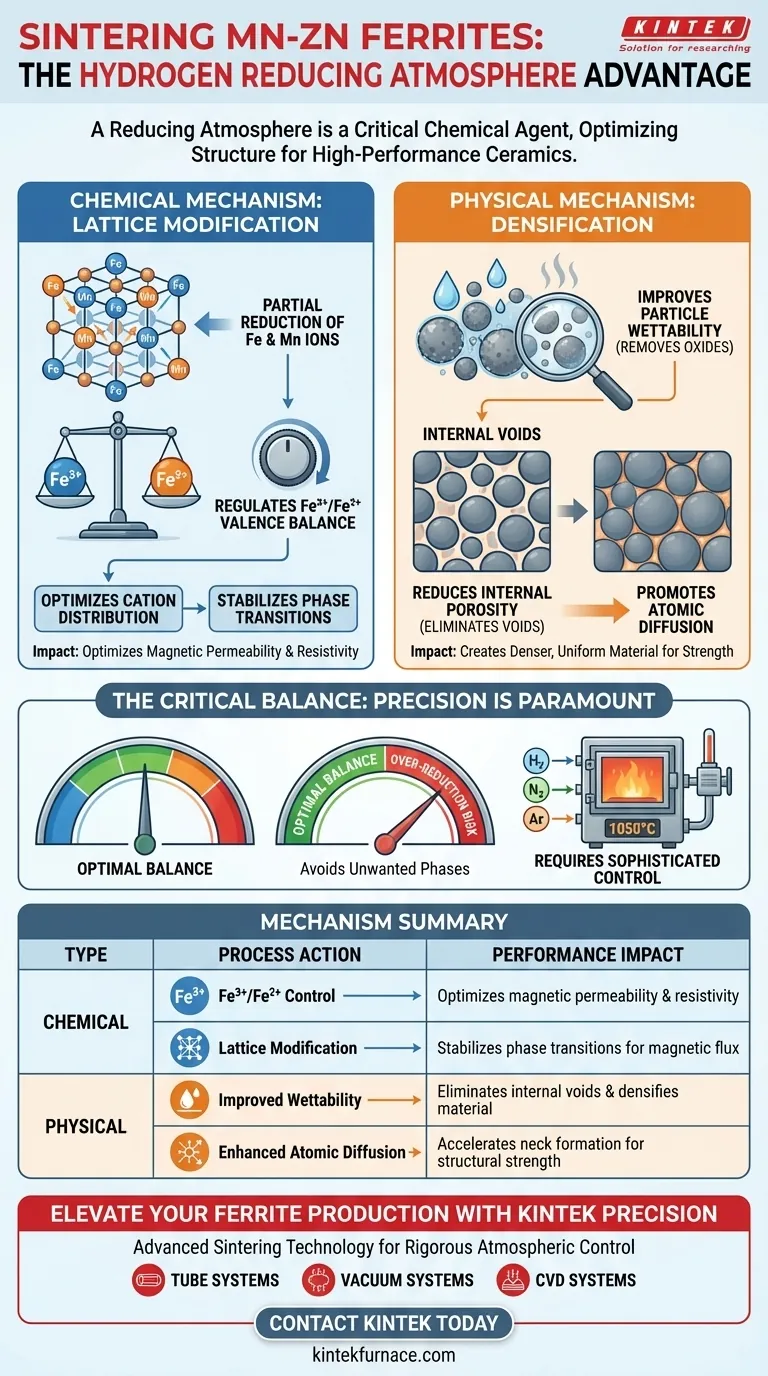
A reducing atmosphere containing hydrogen functions as a critical chemical agent during the high-temperature sintering of Mn-Zn ferrites. It operates by partially reducing iron and manganese ions to modify the cation distribution within the crystal lattice, while simultaneously improving particle wettability to densify the material.
Core Takeaway Achieving high-performance ferrite ceramics is not just about heat; it requires precise chemical control of the material's internal structure. A reducing atmosphere optimizes the Fe3+/Fe2+ valence balance and minimizes porosity, directly translating to superior magnetic permeability and electrical properties.

The Chemical Mechanism: Lattice Modification
Partial Reduction of Ions
In a high-temperature environment (often around 1050°C), hydrogen acts to partially reduce specific metal ions, particularly iron and manganese.
This reduction process is not about removing the metal, but rather adjusting its oxidation state.
Controlling the Valence Balance
This atmosphere allows for the precise regulation of the Fe3+/Fe2+ ratio.
As indicated by the supplementary data, maintaining this specific valence balance is the defining factor for the material's final magnetic permeability and electrical resistivity.
Optimizing Cation Distribution
By altering the oxidation states, the atmosphere modifies how cations are distributed within the ferrite crystal lattice.
This atomic rearrangement is necessary to stabilize the phase transitions required for optimal magnetic performance.
The Physical Mechanism: Densification
Improving Wettability
Beyond chemical changes, the reducing atmosphere significantly improves the wettability of the ferrite particles.
By reacting with and removing surface oxides, the atmosphere "activates" the particle surfaces.
Reducing Internal Porosity
Improved wettability directly promotes the elimination of internal voids.
This reduction in porosity creates a denser, more uniform material structure, which is essential for mechanical strength and consistent magnetic flux.
Promoting Atomic Diffusion
The active reduction of surface films facilitates easier atomic diffusion between particles.
This accelerates the formation of sintering necks, resulting in a more cohesive and structurally sound ceramic body.
Understanding the Trade-offs
The Risk of Over-Reduction
While reduction is necessary, precision is paramount.
An atmosphere that is too strongly reducing can disrupt the delicate stoichiometry of the ferrite, leading to the formation of unwanted phases that degrade magnetic performance.
Complexity of Control
Using hydrogen requires sophisticated equipment, such as a high-temperature tube furnace, to maintain safety and consistency.
You must balance the thermal environment (1050°C) with exact gas flow rates (Argon, Hydrogen, or Nitrogen) to achieve the specific "neutral" or "reducing" window required for Mn-Zn ferrites.
Making the Right Choice for Your Goal
To apply this to your sintering process, you must define your specific performance targets.
- If your primary focus is High-Frequency Performance: Prioritize an atmosphere that targets the specific cation distribution and phase transitions mentioned in the primary reference to minimize eddy current losses.
- If your primary focus is Mechanical Density: Focus on the atmosphere's ability to improve wettability and reduce porosity to ensure a physically robust component.
- If your primary focus is Magnetic Permeability: Tightly control the gas mixture to regulate the Fe3+/Fe2+ ratio, as this chemical balance dictates the magnetic response.
Ultimately, the reducing atmosphere is not just a protective gas; it is an active reactant that defines the electromagnetic identity of your final component.
Summary Table:
| Mechanism Type | Process Action | Performance Impact |
|---|---|---|
| Chemical | Fe3+/Fe2+ Valence Control | Optimizes magnetic permeability & resistivity |
| Chemical | Lattice Modification | Stabilizes phase transitions for magnetic flux |
| Physical | Improved Wettability | Eliminates internal voids & densifies material |
| Physical | Enhanced Atomic Diffusion | Accelerates neck formation for structural strength |
Elevate Your Ferrite Production with KINTEK Precision
Achieving the perfect Fe3+/Fe2+ balance requires more than just heat—it demands rigorous atmospheric control. KINTEK provides the advanced sintering technology needed to master these complex chemical mechanisms.
Backed by expert R&D and world-class manufacturing, we offer high-precision Tube, Vacuum, and CVD systems designed specifically for sensitive sintering processes. Whether you are targeting high-frequency performance or maximum density, our customizable high-temperature lab furnaces ensure the precise gas flow and thermal stability your Mn-Zn ferrites require.
Ready to optimize your material properties? Contact KINTEK today to discuss your custom sintering requirements with our technical team.
Visual Guide
References
- A. Faeghinia. Effects of sintering and pressing conditions on the properties of manganese ferrite. DOI: 10.53063/synsint.2025.53260
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the vacuum capabilities of a controlled atmosphere furnace? Essential for Precise Gas Environment Control
- How does an industrial high-temperature furnace simulate the blast furnace reduction environment? Achieve 30% Reduction
- What is an atmosphere box furnace and what are its primary uses? Essential for Controlled Heat Processing
- What are the key advantages of using atmosphere furnaces? Boost Efficiency and Control in Heat Treatment
- What is the purpose of pre-heating industrial-grade ceramic molds? Ensure Perfect Grain Structures and Casting Yield
- What are the application fields of the box type annealing atmosphere furnace? Essential for Metal, Electronics, and Materials Processing
- What are the primary uses of retort furnaces in industrial settings? Essential for High-Temperature Material Processing
- How does the box type annealing atmosphere furnace ensure accurate temperature control? Discover Precision Heating Solutions



















