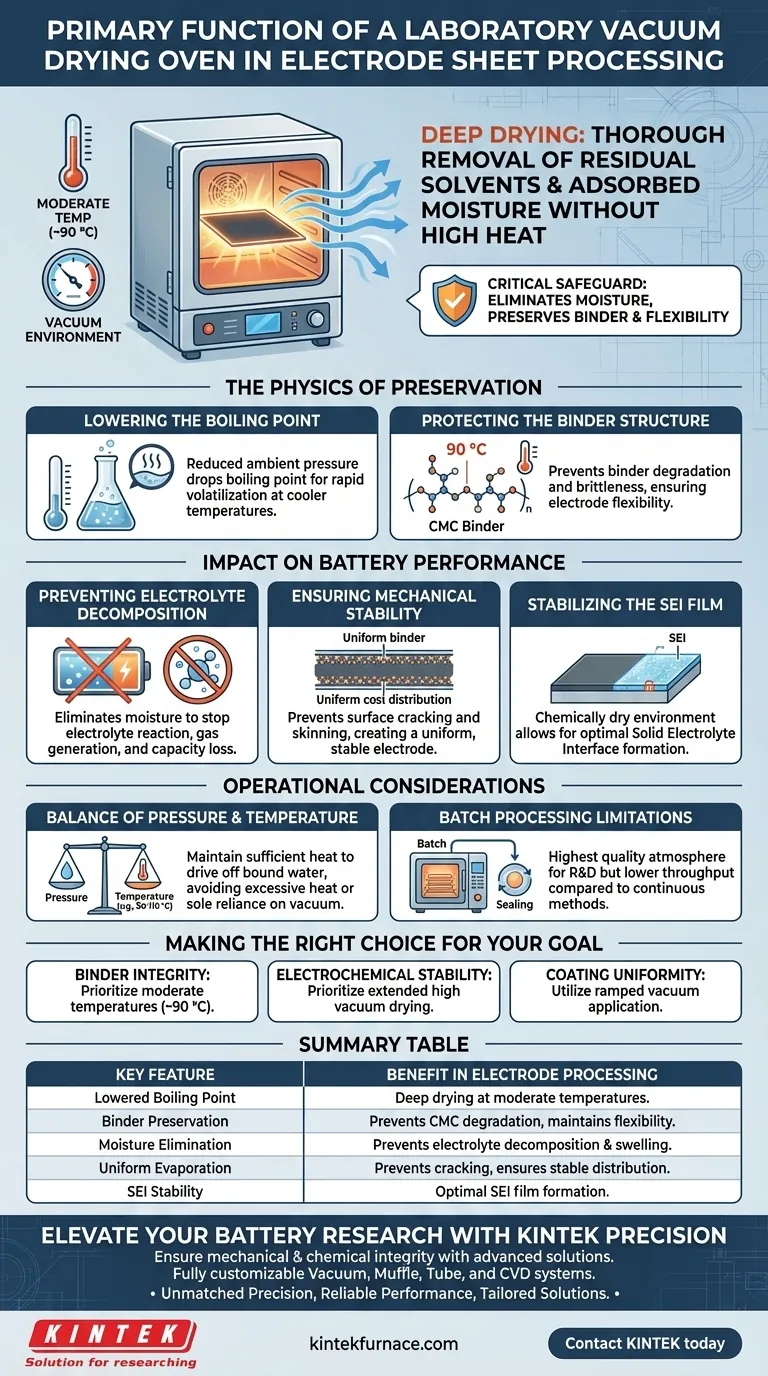
The primary function of a laboratory vacuum drying oven during electrode sheet processing is to thoroughly remove residual solvents and adsorbed moisture from the coated slurry at moderate temperatures. By creating a vacuum environment, the oven significantly lowers the boiling point of liquids, allowing for "deep drying" (typically around 90 °C) without subjecting the delicate binder materials, such as CMC, to destructive high heat.
The vacuum drying process acts as a critical safeguard for battery longevity; it eliminates moisture that would otherwise trigger electrolyte decomposition while preserving the mechanical flexibility of the electrode.

The Physics of Preservation
To understand why this piece of equipment is essential, you must look beyond simple evaporation. The process relies on manipulating pressure to protect the chemical composition of the electrode.
Lowering the Boiling Point
Under standard atmospheric pressure, removing solvents often requires high temperatures that can degrade organic materials.
A vacuum drying oven reduces the ambient pressure surrounding the electrode sheets. This physical change drops the boiling point of residual solvents (and water), enabling rapid volatilization at much cooler temperatures.
Protecting the Binder Structure
The structural integrity of an electrode relies heavily on its binder (often Carboxymethyl Cellulose or CMC).
If exposed to the high heat required for atmospheric drying, these binders can degrade or become brittle. Vacuum drying at controlled temperatures (e.g., 90 °C) removes the solvent while leaving the binder's molecular structure—and therefore the electrode's flexibility—intact.
Impact on Battery Performance
The "deep need" for this equipment stems from the extreme sensitivity of lithium-ion chemistry to contaminants.
Preventing Electrolyte Decomposition
The most critical role of deep drying is the total elimination of moisture.
If residual water remains in the porous electrode structure, it reacts with the battery's electrolyte after assembly. This reaction causes electrolyte decomposition, leading to gas generation (swelling) and detrimental side reactions that permanently reduce battery capacity.
Ensuring Mechanical Stability
Uneven drying can be just as damaging as incomplete drying.
By utilizing negative pressure, the oven prevents the surface layer of the slurry from "skinning over" and drying too quickly, which leads to cracking. This ensures a uniform distribution of the binder between the active material and the current collector, creating a mechanically stable electrode that can withstand cycling.
Stabilizing the SEI Film
Thorough moisture removal is a prerequisite for forming a stable Solid Electrolyte Interface (SEI).
Residual moisture interferes with the initial formation of this protective layer. By ensuring the electrode is chemically dry, the vacuum process allows for a stable SEI to form, which is vital for long-term cycling performance.
Operational Trade-offs
While vacuum drying is superior to air drying for electrodes, it requires precise parameter control.
The Balance of Pressure and Temperature
While vacuum allows for lower temperatures, "lower" is relative. You must still maintain sufficient heat (e.g., 90 °C to 110 °C) to drive off bound water molecules.
Relying solely on vacuum without adequate heat will remove bulk solvents but may leave trace moisture trapped in micropores. Conversely, excessive heat—even under vacuum—can still risk oxidizing surface functional groups or causing binder migration.
Batch Processing Limitations
Laboratory vacuum ovens are typically batch-process units.
Unlike continuous conveyor ovens, they require sealing and pumping down for every cycle. This ensures the highest quality atmosphere for research and development but represents a throughput bottleneck compared to industrial continuous drying methods.
Making the Right Choice for Your Goal
When configuring your drying protocol, your specific research focus dictates your settings.
- If your primary focus is Binder Integrity: Prioritize moderate temperatures (around 90 °C) to protect the CMC structure and prevent electrode brittleness.
- If your primary focus is Electrochemical Stability: Prioritize extended drying times under high vacuum to ensure absolute moisture removal, preventing electrolyte decomposition.
- If your primary focus is Coating Uniformity: Utilize a ramped vacuum application to prevent rapid solvent volatilization that could cause surface cracking.
Success in electrode processing lies in removing contaminants without compromising the delicate chemical architecture of your active materials.
Summary Table:
| Key Feature | Benefit in Electrode Processing |
|---|---|
| Lowered Boiling Point | Facilitates deep drying at moderate temperatures (e.g., 90°C). |
| Binder Preservation | Prevents CMC binder degradation, maintaining electrode flexibility. |
| Moisture Elimination | Prevents electrolyte decomposition and gas generation (swelling). |
| Uniform Evaporation | Prevents surface cracking and ensures stable binder distribution. |
| SEI Stability | Creates a chemically dry environment for optimal SEI film formation. |
Elevate Your Battery Research with KINTEK Precision
Ensure the mechanical and chemical integrity of your electrodes with our advanced laboratory solutions. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Vacuum, Muffle, Tube, and CVD systems—all fully customizable to meet the rigorous demands of battery development and high-temp material science.
Our value to you:
- Unmatched Precision: Maintain the perfect balance of pressure and temperature to protect delicate binders.
- Reliable Performance: Eliminate trace moisture to prevent electrolyte decomposition.
- Tailored Solutions: From R&D vacuum ovens to industrial-grade furnaces, we build to your specs.
Contact KINTEK today to optimize your electrode drying protocol
Visual Guide
References
- Jianjiao Wang. An S-Infused/S, F-Codoped PVDF-Derived Carbon as a High-Performance Anode for Sodium-Ion Batteries. DOI: 10.3390/ma18174018
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a vacuum heat treatment furnace necessary for the gas nitriding of AISI 5140 steel? Achieve Precision Hardening
- What are the advantages of vacuum furnaces for sintering? Achieve Superior Material Quality and Control
- How are vacuum brazing challenges overcome in furnace design? Master Precision and Purity for Strong Joints
- What is vacuum hardening? Achieve Superior Hardness with Pristine Surface Finish
- How does temperature control precision of industrial melting furnaces affect intermetallic phase selection?
- What factors should be considered when choosing between argon and nitrogen for vacuum furnace applications? Optimize Your Heat Treatment Process
- How is the heating chamber of a vacuum annealing furnace constructed? Optimize Your Material Processing
- What is the significance of vacuum furnaces in powder metallurgy? Achieve High-Purity, Dense Metal Parts



















