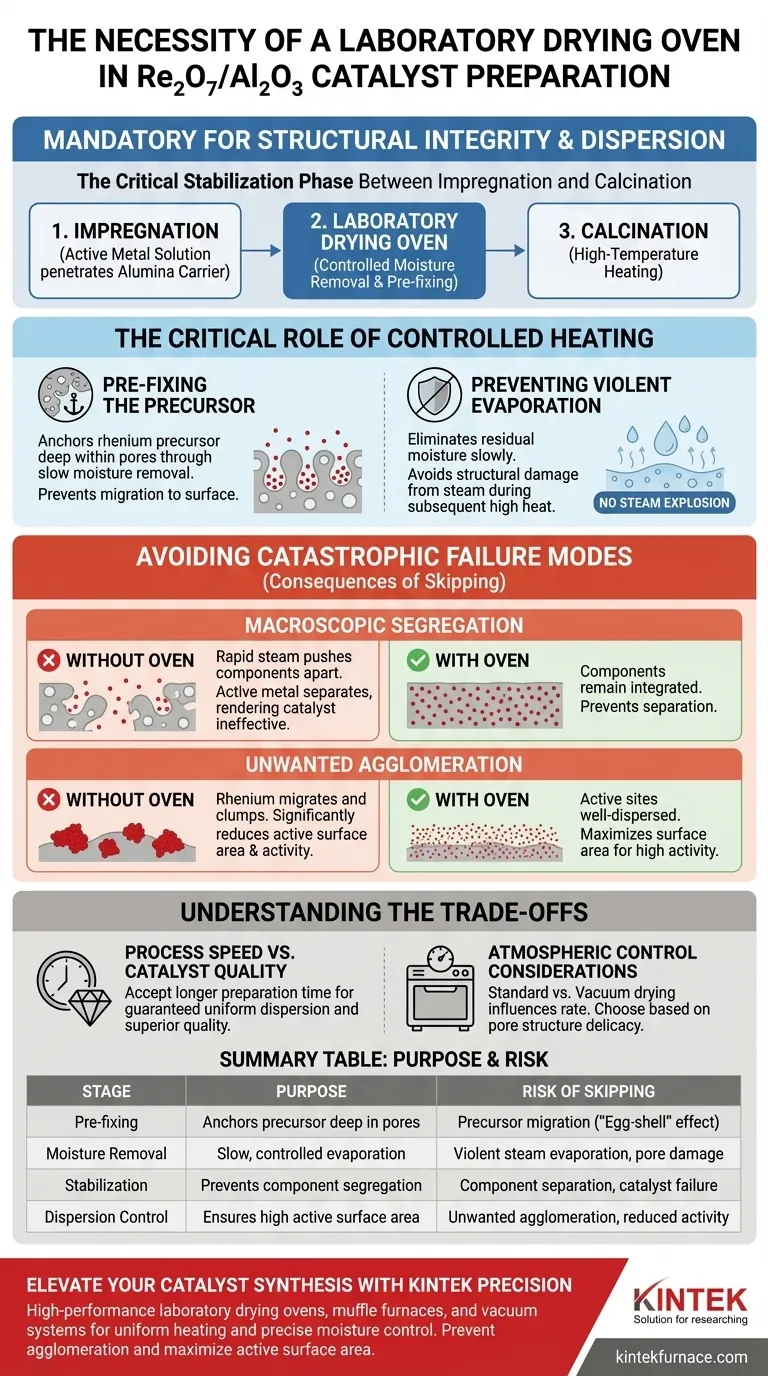
The use of a laboratory drying oven is mandatory in the preparation of supported Re2O7/Al2O3 catalysts to ensure the structural integrity and dispersion of the active metal. By treating the rhenium-loaded alumina carrier in this stable environment, you facilitate the controlled removal of moisture. This specific step pre-fixes the rhenium precursor within the pores of the carrier, which is essential for the catalyst's final performance.
Core Takeaway The drying oven serves as a critical stabilization phase between impregnation and calcination. Its primary function is to anchor the rhenium precursor inside the alumina pores through slow moisture removal, preventing the structural damage and metal clumping that occur with rapid heating.

The Critical Role of Controlled Heating
Pre-fixing the Precursor
The impregnation method relies on the active metal solution penetrating the porous structure of the alumina carrier.
The drying oven does more than simply dry the material; it "pre-fixes" the rhenium precursor in place.
By removing water slowly, the rhenium remains deposited deep within the pores rather than being drawn to the surface by rapid evaporation.
Preventing Violent Evaporation
The subsequent step in catalyst preparation involves high-temperature calcination.
If the carrier is not thoroughly dried first, residual moisture will evaporate violently when exposed to calcination temperatures.
The drying oven eliminates this moisture in a stable environment, ensuring the transition to high heat is safe for the material's microstructure.
Avoiding Catastrophic Failure Modes
Stopping Macroscopic Segregation
One of the primary risks in preparing Re2O7/Al2O3 catalysts is the separation of components.
Without the controlled drying phase, the rapid exit of steam during calcination can physically push the rhenium components apart.
This leads to "macroscopic segregation," where the active metal separates from the carrier, rendering the catalyst ineffective.
Preventing Unwanted Agglomeration
For a catalyst to function correctly, the active sites must be well-dispersed.
Bypassing the drying oven often causes the rhenium components to migrate and clump together.
This "unwanted agglomeration" reduces the surface area of the active metal, significantly degrading the catalytic activity.
Understanding the Trade-offs
Process Speed vs. Catalyst Quality
Using a laboratory drying oven is a time-intensive step compared to rapid drying methods.
While faster drying techniques exist, they often lead to the "egg-shell" effect or uneven distribution of the metal.
You must accept the trade-off of a longer preparation time to guarantee the uniform dispersion of the rhenium.
Atmospheric Control Considerations
While standard ovens are effective, the specific type of oven (blast vs. vacuum) can influence the drying rate.
A standard drying oven ensures consistent circulation, but it operates at atmospheric pressure.
If the pore structure is extremely delicate, one might consider vacuum drying to lower the boiling point, though this changes the penetration profile of the metal.
Making the Right Choice for Your Goal
To ensure your Re2O7/Al2O3 catalyst performs as intended, apply the following guidelines:
- If your primary focus is maximizing active surface area: Prioritize the drying oven step to ensure high dispersion and prevent the agglomeration of rhenium particles.
- If your primary focus is structural stability: Use the drying oven to remove all moisture before calcination to prevent pore damage caused by violent steam evaporation.
Skipping the drying oven is not a time-saver; it is a direct path to a segregated, low-performance catalyst.
Summary Table:
| Stage | Purpose | Risk of Skipping |
|---|---|---|
| Pre-fixing | Anchors rhenium precursors deep within alumina pores | Precursor migration to surface ("Egg-shell" effect) |
| Moisture Removal | Slow, controlled evaporation of residual solvent | Violent steam evaporation and pore structural damage |
| Stabilization | Prevents macroscopic segregation of components | Component separation and catalyst failure |
| Dispersion Control | Ensures high active metal surface area | Unwanted metal agglomeration and reduced activity |
Elevate Your Catalyst Synthesis with KINTEK Precision
Don’t let improper drying compromise your catalyst's performance. Backed by expert R&D and manufacturing, KINTEK offers high-performance laboratory drying ovens, muffle furnaces, and vacuum systems designed to ensure uniform heating and precise moisture control for delicate impregnation processes.
Whether you need standard drying or customizable high-temperature systems, our equipment is engineered to prevent agglomeration and maximize active surface area for your Re2O7/Al2O3 catalysts and beyond.
Ready to optimize your lab's efficiency? Contact us today to find the perfect thermal solution for your unique research needs!
Visual Guide
References
- Joanna Malarz, Katarzyna Leszczyńska-Sejda. Research on the Production of Methyltrioxorhenium and Heterogenous Catalysts from Waste Materials. DOI: 10.3390/cryst15080717
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does the high-temperature boiling step play in rice husk silica conversion? Boost Your Extraction Yields
- What is the objective of GC-MS analysis on bio-oil? Unlock Chemical Value and Industrial Utility
- What role does the impregnation method play when using cordierite as a carrier? Enhance Catalyst Loading & Activity
- How do segmented heating and cooling cycles affect the microwave-assisted synthesis of 2D iron oxide (Fe2O3)?
- What is the function of a solvothermal reactor? Optimize Carbon Polymer Dots (CPDs) Synthesis with Precision Pressure
- What are the technical characteristics of Physical Vapor Deposition (PVD) equipment for perovskite? Precision Thin Films
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth
- How does an autoclave assist in modifying bio-carbon with cobalt oxide? Unlock High-Performance Nano-Composites



















