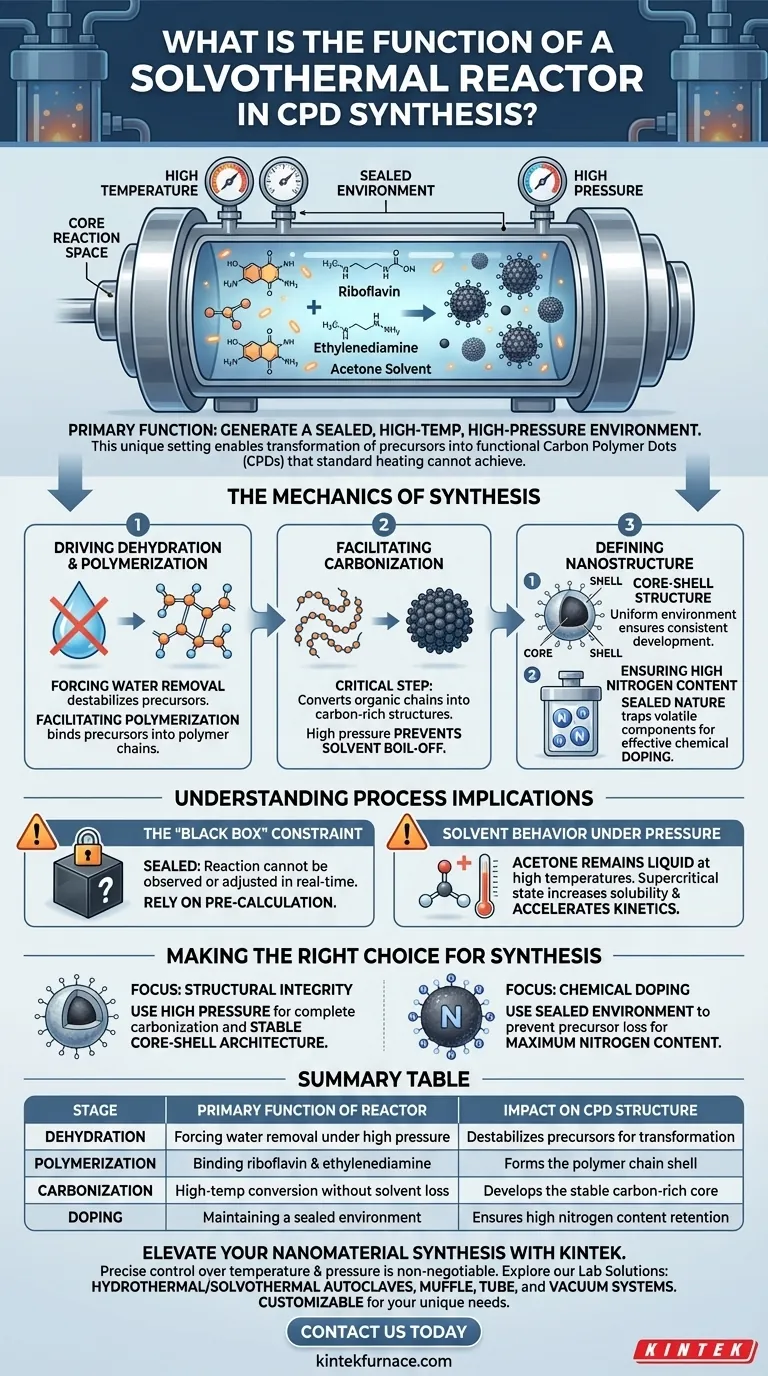
The primary function of a solvothermal reactor is to generate a sealed, high-temperature, and high-pressure environment that serves as the core reaction space for synthesizing Carbon Polymer Dots (CPDs). By confining reactants—specifically riboflavin and ethylenediamine in an acetone solvent—this vessel creates conditions that standard atmospheric heating cannot achieve. It is this unique environment that enables the transformation of precursor molecules into functional nanoparticles.
The solvothermal reactor is the catalyst for forcing dehydration, polymerization, and carbonization under pressure. This mechanism is essential for engineering the specific core-shell structure and high nitrogen content required for high-quality Carbon Polymer Dots.
The Mechanics of Synthesis
The solvothermal reactor does more than simply heat the mixture; it fundamentally alters how the chemical reaction proceeds by trapping pressure and preventing solvent evaporation.
Driving Dehydration and Polymerization
The process begins by forcing the dehydration of the precursor molecules. This removal of water molecules is the first step in destabilizing the raw materials to prepare them for transformation.
Simultaneously, the reactor facilitates polymerization. Under these intense conditions, the riboflavin and ethylenediamine molecules bind together to form longer polymer chains.
Facilitating Carbonization
Once polymerization is underway, the reactor facilitates carbonization. This is the critical step where the organic polymer chains are converted into the carbon-rich structures necessary for "dot" formation.
Without the high pressure maintained by the reactor, the temperature required to achieve this carbonization would likely cause the solvent to boil away before the reaction completes.
Defining the Nanostructure
The physical constraint of the reactor directly influences the architecture of the final nanoparticle.
Forming the Core-Shell Structure
The primary reference indicates that this method is required to form nanoparticles with a specific core-shell structure.
The "core" typically consists of the carbonized material, while the "shell" retains functional groups from the polymerization phase. The reactor’s uniform environment helps ensure this structure develops consistently across the batch.
Ensuring High Nitrogen Content
The sealed nature of the reactor is vital for chemical doping. By preventing the escape of volatile components, the reactor ensures that the nitrogen content from the ethylenediamine is effectively incorporated into the final CPD structure.
Understanding the Process Implications
While the solvothermal reactor is effective, it introduces specific constraints that must be managed during the experimental process.
The "Black Box" Constraint
Because the reactor must remain sealed to maintain pressure, the reaction cannot be observed or adjusted in real-time.
You must rely entirely on the initial reactant ratios and temperature settings to drive the process to completion, making precise pre-calculation of the riboflavin and ethylenediamine mixture critical.
Solvent Behavior Under Pressure
The use of acetone as a solvent is notable because it has a relatively low boiling point.
The reactor allows the acetone to remain liquid at temperatures far exceeding its normal boiling point. This supercritical or near-supercritical state increases the solubility of the precursors and accelerates the reaction kinetics.
Making the Right Choice for Your Synthesis
To maximize the quality of your Carbon Polymer Dots, you must align the reactor's capabilities with your specific material goals.
- If your primary focus is structural integrity: Rely on the solvothermal reactor's high pressure to drive the complete carbonization necessary for a stable core-shell architecture.
- If your primary focus is chemical doping: Use the sealed environment to prevent the loss of volatile precursors, ensuring the maximum possible nitrogen content in the final product.
The solvothermal reactor provides the essential thermodynamic container required to turn simple organic precursors into complex, high-performance nanoparticles.
Summary Table:
| Stage of Synthesis | Primary Function of Reactor | Impact on CPD Structure |
|---|---|---|
| Dehydration | Forcing water removal under high pressure | Destabilizes precursors for transformation |
| Polymerization | Binding riboflavin & ethylenediamine | Forms the polymer chain shell |
| Carbonization | High-temp conversion without solvent loss | Develops the stable carbon-rich core |
| Doping | Maintaining a sealed environment | Ensures high nitrogen content retention |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise control over temperature and pressure is non-negotiable for high-performance Carbon Polymer Dots. Backed by expert R&D and manufacturing, KINTEK offers a wide range of lab solutions, including Hydrothermal/Solvothermal Autoclaves, Muffle, Tube, and Vacuum systems, all customizable to meet your unique chemical synthesis needs.
Don't let volatile precursors escape—ensure consistent core-shell structures and optimal doping every time. Contact us today to find the perfect reactor for your lab!
Visual Guide
References
- Zoran Marković, Biljana M. Todorović Marković. Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films. DOI: 10.3390/jfb15030073
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Laboratory Muffle Oven Furnace with Bottom Lifting
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- Why does high-phenyl conductive silicone rubber require secondary vulcanization? Essential Stability Guide
- What role does an RTA system play in Zirconia preparation? Master Phase Transformation for Advanced Deposition
- How do high-pressure reaction environments facilitate the solvothermal synthesis of Ag2Se? Precision Phase Control
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- What role does an oscillating heating stage play in WO3 thin film growth? Control Kinetics and Crystal Orientation
- How does a graphite furnace work? Achieve Ultra-Trace Element Analysis
- How does a stable constant temperature environment influence the structural development of LDHs during aging?
- Why is a vacuum desiccator used for the preservation of extracted fruit peel extracts? Protect Bioactive Compounds



















