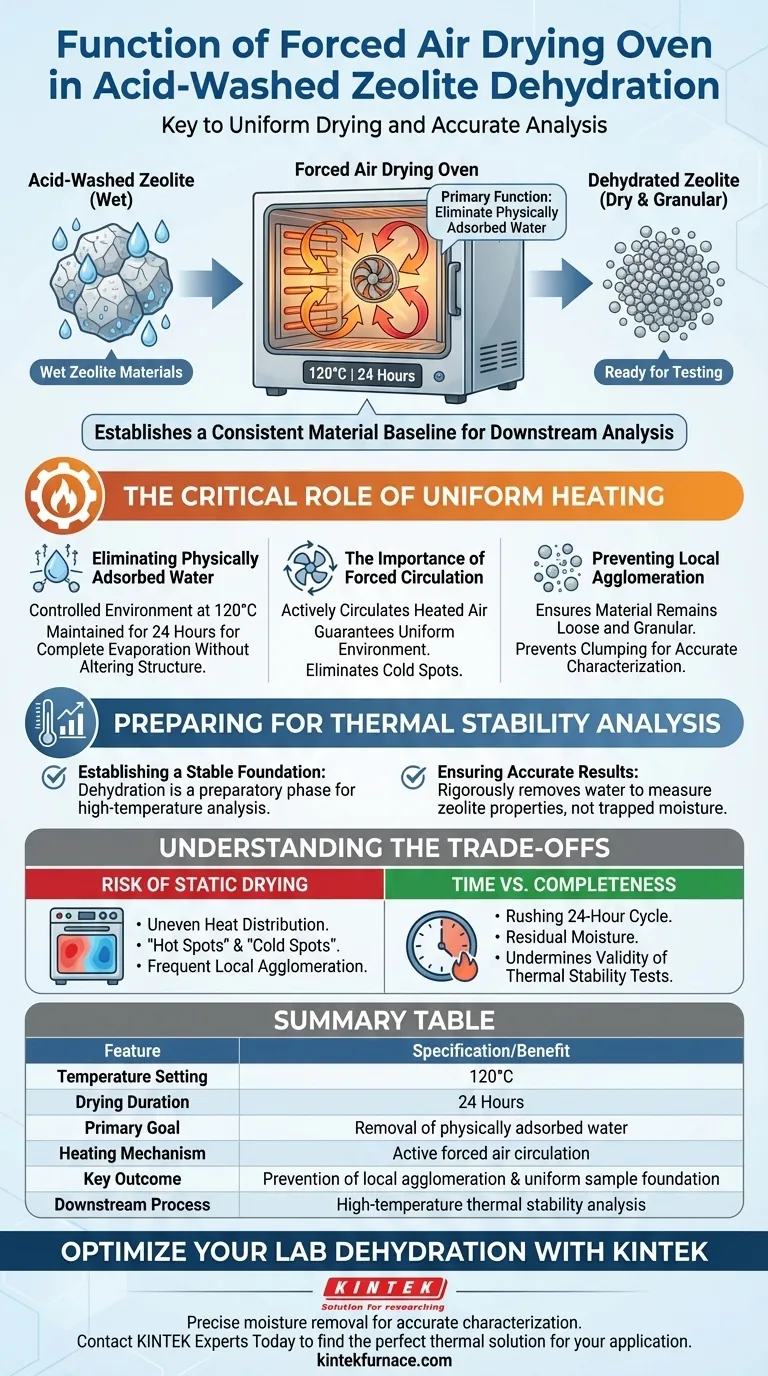
The primary function of a forced air drying oven in this process is to eliminate physically adsorbed water from acid-washed zeolite materials to prepare them for further testing. By maintaining a constant temperature of 120 degrees Celsius for a duration of 24 hours, the oven ensures the material is thoroughly dried. This specific dehydration step creates the stable material baseline required for accurate downstream analysis.
The forced air mechanism is essential not just for drying, but for ensuring a uniform heating environment that prevents material clumping (agglomeration), thereby establishing a consistent foundation for high-temperature thermal stability analysis.

The Critical Role of Uniform Heating
Eliminating Physically Adsorbed Water
The first objective of this stage is the removal of moisture that has physically adhered to the surface of the zeolite after washing.
To achieve this, the oven creates a controlled environment at 120 degrees Celsius.
This temperature is maintained strictly for 24 hours to ensure complete evaporation of surface moisture without altering the chemical structure of the zeolite.
The Importance of Forced Circulation
Unlike static ovens, a forced air oven actively circulates heated air throughout the chamber.
This circulation guarantees a uniform heating environment for the entire sample batch.
It eliminates cold spots or uneven temperature gradients that could lead to inconsistent drying rates across the material.
Preventing Local Agglomeration
A critical advantage of uniform heating is the prevention of local agglomeration.
When heating is uneven, particles can clump together, altering the physical consistency of the zeolite.
Forced air ensures that the material remains loose and granular, which is vital for accurate physical characterization.
Preparing for Thermal Stability Analysis
Establishing a Stable Foundation
The dehydration stage is not the final step; it is a preparatory phase for high-temperature thermal stability analysis.
Any remaining physically adsorbed water can interfere with thermal analysis data, leading to skewed results.
By rigorously removing this water, the oven ensures that subsequent tests measure the properties of the zeolite itself, not the behavior of trapped moisture.
Understanding the Trade-offs
The Risk of Static Drying
Using an oven without forced air circulation introduces significant risks to the sample integrity.
Without air movement, heat distribution becomes uneven, leading to "hot spots" and "cold spots" within the sample tray.
This unevenness frequently causes local agglomeration, where parts of the sample clump together while others remain loose.
Time vs. Completeness
The 24-hour cycle at 120 degrees Celsius is a time-intensive process that cannot be rushed.
Shortening this duration may leave residual moisture in the pore structure of the zeolite.
Incomplete drying undermines the validity of future thermal stability tests, rendering the data unreliable.
Ensuring Process Integrity for Your Project
To maximize the reliability of your zeolite characterization, consider the following recommendations based on your specific objectives:
- If your primary focus is Sample Homogeneity: Prioritize forced air circulation to prevent agglomeration and ensure the material remains granular and uniform.
- If your primary focus is Analytical Accuracy: Strictly adhere to the 24-hour drying period at 120°C to fully remove physically adsorbed water before thermal stability testing.
Consistency in the drying stage is the invisible variable that dictates the quality of your final analytical data.
Summary Table:
| Feature | Specification/Benefit |
|---|---|
| Temperature Setting | 120°C |
| Drying Duration | 24 Hours |
| Primary Goal | Removal of physically adsorbed water |
| Heating Mechanism | Active forced air circulation |
| Key Outcome | Prevention of local agglomeration & uniform sample foundation |
| Downstream Process | High-temperature thermal stability analysis |
Optimize Your Lab Dehydration with KINTEK
Precise moisture removal is the cornerstone of accurate zeolite characterization. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, alongside specialized forced air drying ovens. Our lab high-temp furnaces are fully customizable to meet your unique thermal processing needs, ensuring uniform heat distribution and reliable data integrity for your research.
Ready to enhance your lab's efficiency?
Contact KINTEK Experts Today to find the perfect thermal solution for your application.
Visual Guide
References
- Sandugash Tanirbergenova, З. А. Мансуров. Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit. DOI: 10.3390/pr13092896
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How does a vacuum pressure infiltration system contribute to Diamond/Cu composite green bodies? Achieve 60% Density
- What is the significance of preheating UHPC molds? Ensure Safety & Longevity with High-Temp Furnaces
- Process conditions for HEA cladding thermal experiments: Ensuring 800°C stability and 1680-hour endurance.
- Why is a constant temperature oven required for CoCrFeNiMn alloy powders? Ensure Superior Defect-Free Deposition
- What role does thermal processing in a furnace play in phase analysis of kaolin? Optimize Your Catalyst Structure
- Why is the mechanical mixing of precursor powders necessary for ITO thin films? Guide to Precision Growth
- Why is vacuum sealing technology essential for K2In2As3 synthesis? Master High-Purity Solid-State Reactions
- Why is a constant temperature water bath or hot plate required for MXene post-treatment? Master Precise Delamination



















