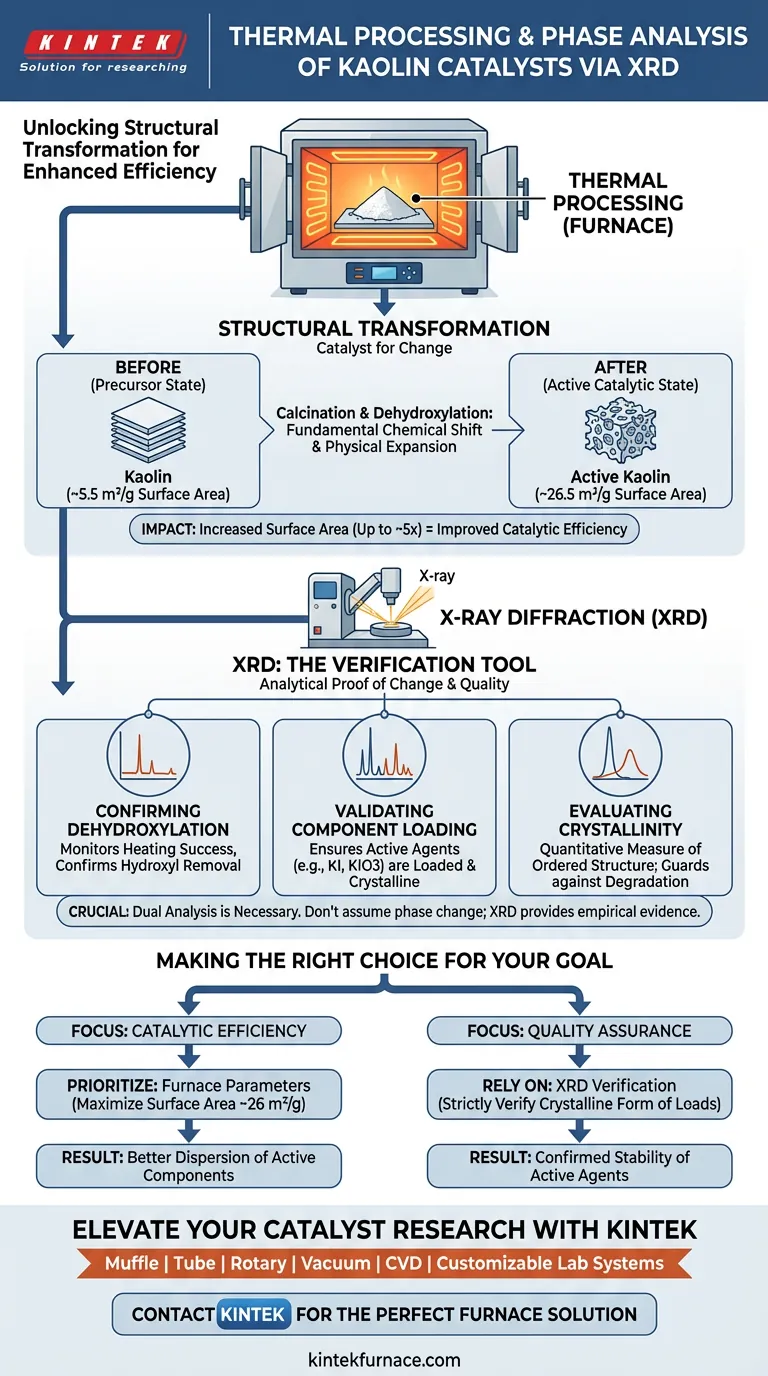
Thermal processing serves as the catalyst for structural transformation, acting as the physical mechanism that alters the properties of kaolin. In this workflow, the furnace induces critical changes such as dehydroxylation and phase transitions, while X-ray diffraction (XRD) functions as the verification tool to confirm these changes have occurred and to validate the crystalline state of active components.
The furnace provides the high-temperature environment necessary to physically evolve the kaolin structure, while XRD provides the analytical proof that essential phase transitions and the loading of active agents like KI or KIO3 have successfully taken place.
The Mechanism of Structural Transformation
Inducing Phase Transitions
The primary function of the high-temperature furnace is to drive calcination. This process forces the kaolin to undergo dehydroxylation, fundamentally changing its chemical structure. Without this thermal energy, the material would remain in its precursor state and lack the necessary catalytic properties.
Enhancing Physical Architecture
Beyond chemical changes, thermal processing dramatically alters the physical architecture of the material. Calcination significantly increases the specific surface area of the kaolin.
The Impact on Efficiency
Data indicates that proper thermal treatment can expand the surface area from approximately 5.514 m²/g to 26.567 m²/g. This physical expansion is crucial because it creates a larger interface for chemical reactions, directly correlating to improved catalytic efficiency.
The Role of XRD as a Verification Tool
Confirming Dehydroxylation
XRD is utilized to monitor the success of the heating process. It analyzes the diffraction patterns to confirm that the hydroxyl groups have been removed and that the intended phase transition is complete.
Validating Component Loading
For kaolin catalysts loaded with active components, such as KI (Potassium Iodide) or KIO3 (Potassium Iodate), XRD is essential for quality control. It verifies that these components are not only present but have been loaded in their required crystalline states.
Evaluating Crystallinity
XRD provides a quantitative measure of crystallinity. This allows researchers to ensure that the thermal processing was sufficient to create an ordered structure without degrading the active components.
Understanding the Analytical Trade-offs
Structural Change vs. Detection
While the furnace increases surface area (measured by BET analysis), XRD focuses on crystalline order. It is important to recognize that XRD may not fully characterize amorphous regions created during thermal processing.
The Necessity of Dual Analysis
Reliance on thermal processing logs alone is insufficient. You cannot assume a phase change occurred simply because the furnace reached a set temperature; XRD provides the empirical evidence that the internal structure actually shifted as predicted.
Making the Right Choice for Your Goal
To optimize your catalyst preparation and analysis, consider the following specific objectives:
- If your primary focus is Catalytic Efficiency: Prioritize the furnace parameters to maximize surface area expansion (aiming for the ~26 m²/g benchmark) to ensure better dispersion of active components.
- If your primary focus is Quality Assurance: Rely on XRD to strictly verify that active loads like KI or KIO3 have retained their specific crystalline forms after the high-temperature treatment.
By combining precise thermal control with rigorous phase analysis, you ensure the physical structure supports the chemical function.
Summary Table:
| Process Step | Mechanism | Impact on Kaolin Structure | Verification Method |
|---|---|---|---|
| Calcination | Thermal Dehydroxylation | Expands surface area from ~5.5 to ~26.5 m²/g | XRD Pattern Analysis |
| Phase Transition | Structural Evolution | Fundamental shift to active catalytic state | Peak Intensity & Shift |
| Component Loading | Thermal Incorporation | Fixes active agents (KI/KIO3) in crystalline state | XRD Crystallinity Check |
Elevate Your Catalyst Research with KINTEK
Precise phase transitions in kaolin catalysts demand exact temperature control and high-performance equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temperature furnaces—all fully customizable to meet your unique research needs.
Whether you are aiming to maximize specific surface area or ensure the crystalline stability of active components, our advanced heating solutions provide the reliability your lab requires.
Contact us today to find the perfect furnace for your application!
Visual Guide
References
- Luqman Buchori, Ndaru Okvitarini. Preparation of KI/KIO3/Methoxide Kaolin Catalyst and Performance Test of Catalysis in Biodiesel Production. DOI: 10.26554/sti.2024.9.2.359-370
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a rotary evaporator contribute to the concentration phase of TiO2 and ZrO2 pastes? Achieve Precision Viscosity
- What are the advantages of using a vacuum drying oven for magnesium slag? Preserving Sample Integrity
- Why are raw materials compacted into briquettes for vacuum carbothermal reduction? Optimize Your Magnesium Production
- Why is vacuum freeze-drying necessary for FeNC/MXene catalysts? Preserving 2D Architecture for Peak Performance
- How is mechanochemical grinding used in lithium battery recovery? Unlock Efficient Solid-State Material Repair
- Which furnace is used for sintering? Find the Right High-Temperature Solution for Your Materials
- How does a cooling circulation unit assist in plastic pyrolysis? Optimize Bio-Oil & Gas Separation
- Why is a constant temperature drying oven used at 120°C for 16 hours for NiCuCe catalysts? Optimize Site Dispersion



















