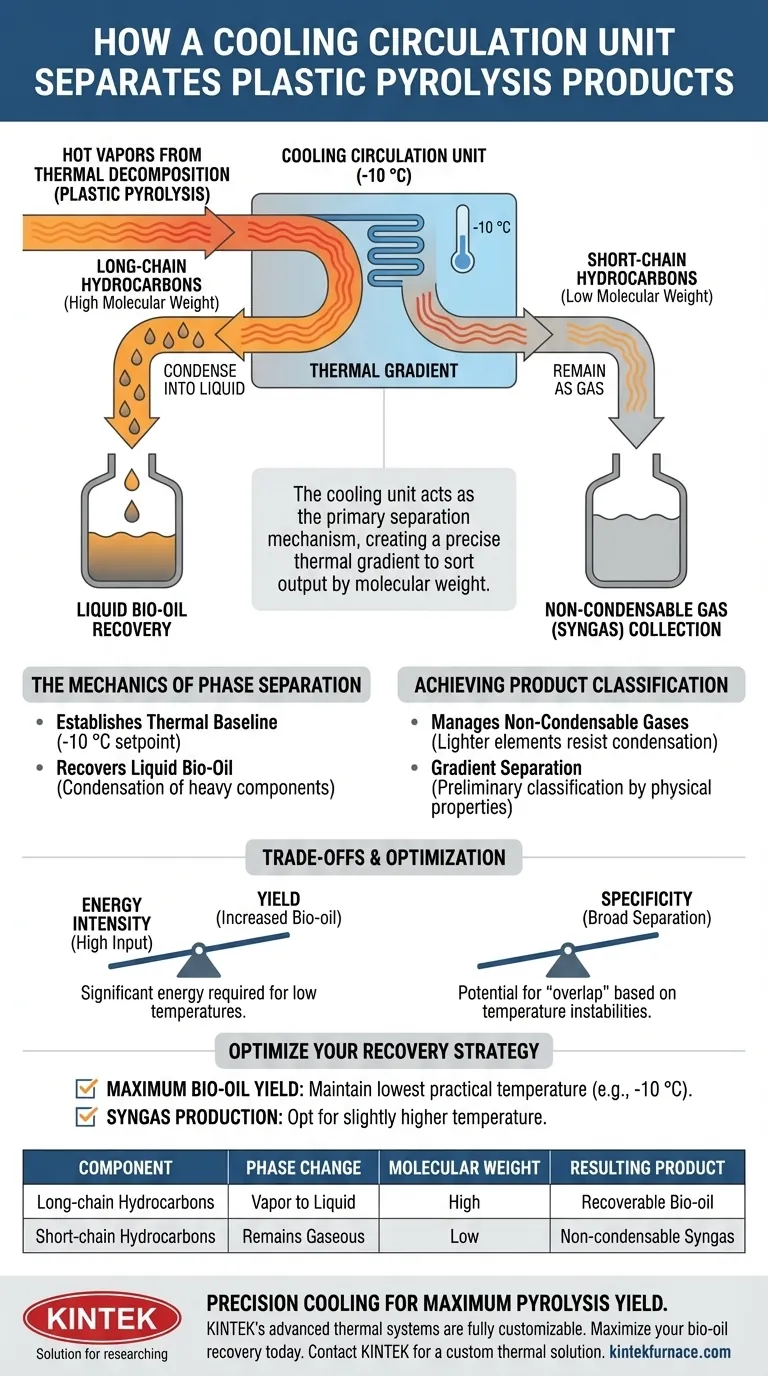
The cooling circulation unit acts as the primary separation mechanism within a plastic pyrolysis system. It functions by strictly maintaining a low condensation temperature, such as -10 °C, to process the hot vapors generated during thermal decomposition. By creating this thermal environment, the unit forces heavier long-chain hydrocarbons to condense into liquid bio-oil while permitting lighter short-chain hydrocarbons to remain as gas, effectively sorting the output by molecular weight.
While heat breaks the plastic down, the cooling unit determines the final product form. By establishing a precise thermal gradient, it transforms a mixed vapor stream into distinct, recoverable resources—liquid bio-oil and combustible gas.

The Mechanics of Phase Separation
Establishing the Thermal Baseline
The core function of the cooling circulation unit is to maintain a consistent, low-temperature environment. By holding the system at a specific setpoint, such as -10 °C, it creates a drastic temperature difference relative to the incoming hot vapors.
Recovering Liquid Bio-Oil
When the thermal decomposition vapors contact this cooled environment, heavier components react immediately. These long-chain hydrocarbons lose thermal energy and condense from a vapor into a liquid state. This phase change allows for the direct recovery of bio-oil.
Achieving Product Classification
Managing Non-Condensable Gases
Not all components react to the cold environment in the same way. Lighter, short-chain hydrocarbons have boiling points that remain below the unit's operating temperature. Consequently, these elements resist condensation and pass through the system as non-condensable gases.
Gradient Separation
This process creates a "gradient separation" of the volatile components. Rather than producing a mixed slurry, the unit performs a preliminary classification. It automatically sorts the output into liquid and gas streams based on the physical properties of the hydrocarbon chains.
Understanding the Trade-offs
Energy Intensity vs. Yield
maintaining a temperature as low as -10 °C requires significant energy input for the circulation system. Operators must carefully calculate whether the increased yield of liquid bio-oil justifies the energy cost of maintaining such low temperatures.
Specificity of Separation
While effective for preliminary classification, a single cooling stage provides a broad separation rather than precise chemical isolation. There is a potential for "overlap," where medium-weight chains may fluctuate between gas and liquid phases depending on minor temperature instabilities.
Optimizing Your Recovery Strategy
To maximize the efficiency of your pyrolysis system, align your cooling strategy with your specific production goals:
- If your primary focus is Maximum Bio-oil Yield: Ensure your cooling unit can consistently maintain the lowest practical temperature (e.g., -10 °C) to force condensation of even lighter liquid fractions.
- If your primary focus is Syngas Production: You may opt for a slightly higher condensation temperature, allowing more medium-chain hydrocarbons to remain in the gaseous state for downstream combustion.
Precise thermal management in the cooling stage is the difference between a raw vapor stream and a valuable, fractionated product inventory.
Summary Table:
| Component | Phase Change | Molecular Weight | Resulting Product |
|---|---|---|---|
| Long-chain Hydrocarbons | Vapor to Liquid | High | Recoverable Bio-oil |
| Short-chain Hydrocarbons | Remains Gaseous | Low | Non-condensable Syngas |
| Cooling Setpoint (-10°C) | Thermal Catalyst | N/A | High-Yield Condensation |
| Thermal Gradient | Separation Force | N/A | Product Classification |
Precision Cooling for Maximum Pyrolysis Yield
Don't let valuable hydrocarbons escape as waste. KINTEK’s advanced thermal systems are backed by expert R&D and manufacturing to ensure your plastic pyrolysis process is as efficient as possible. Whether you need a Muffle, Tube, or Vacuum furnace system, our equipment is fully customizable to your unique temperature gradients and recovery goals.
Maximize your bio-oil recovery today. Contact KINTEK for a custom thermal solution and leverage our expertise in high-temperature lab equipment for your next project.
Visual Guide
References
- Wei Xiong, Jun Zhao. Acidic Site-Controlled ZSM-5 Catalysts for Fast Molten-Phase Pyrolysis of Plastic Waste with Tunable Product Distribution. DOI: 10.1021/acs.energyfuels.5c02781
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the technical value of using a vacuum drying oven? Master Platinum Catalyst Precision and Activity
- How does industrial-scale forging equipment influence the morphology of primary carbonitrides in H13 tool steel?
- Why is precise superheat temperature control required? Unlock High-Quality Soft Magnetic Nanocrystalline Alloys
- Flash Pyrolyser vs. TGA: Which is Best for Assessing RDF as a Blast Furnace Reducing Agent?
- What is the role of an industrial high-speed ball mill in kaolin pretreatment? Enhance Reactivity & Surface Area
- What are the applications of heat treatment furnaces in the aerospace industry? Enhance Component Performance for Extreme Conditions
- Why is a heating furnace with high-precision temperature control required for alpha-Fe2O3/FeOOH? Expert Synthesis Guide
- What is the function of a laboratory drying oven in the preparation of solid bismuth molybdate materials? Expert Tips



















