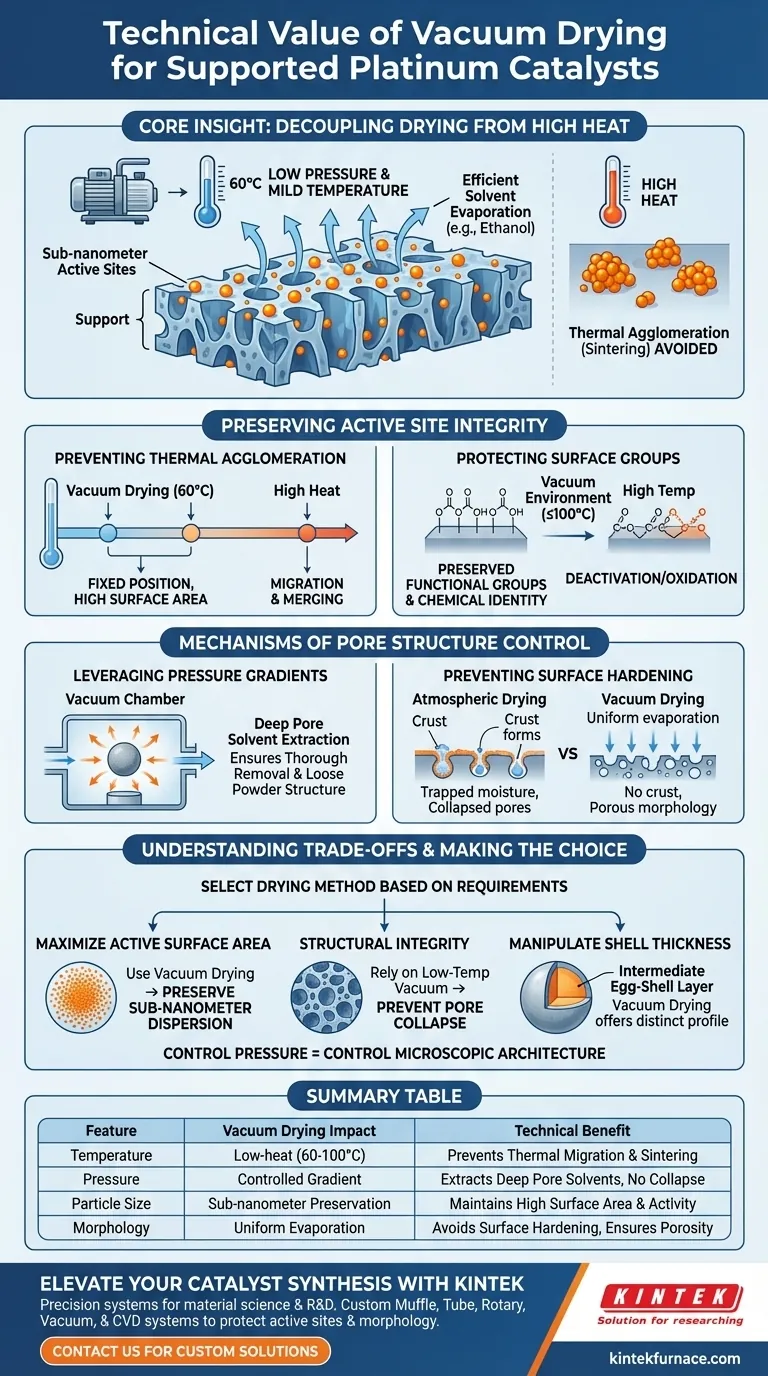
The primary technical value of a vacuum drying oven in processing supported platinum catalysts is its ability to facilitate thorough solvent evaporation at significantly reduced temperatures. By lowering the environmental pressure, you can remove organic solvents like ethanol at mild conditions (e.g., 60 °C), effectively decoupling the drying process from the high thermal energy usually required. This prevents the thermal migration or agglomeration of platinum particles, ensuring the preservation of sub-nanometer active sites.
Core Insight: The critical challenge in catalyst preparation is removing solvents without altering the delicate microstructure of the metal and support. Vacuum drying solves this by utilizing low pressure rather than high heat, protecting the original distribution of active sites while preventing the collapse of the support’s morphology.

Preserving Active Site Integrity
Preventing Thermal Agglomeration
High temperatures are the enemy of nano-dispersed catalysts. When excessive heat is applied during drying, platinum particles gain enough energy to migrate across the support surface and merge, a process known as sintering.
Vacuum drying mitigates this by operating at temperatures as low as 60 °C. This low-thermal environment ensures that the platinum remains fixed in its original position, maintaining the high surface area of sub-nanometer active sites essential for catalytic performance.
Protecting Surface Functional Groups
Beyond the metal particles, the carbon support itself often contains heat-sensitive functional groups. High-temperature drying can deactivate these groups or cause the oxidation of organic-inorganic hybrid precursors.
The vacuum environment allows for rapid drying at temperatures (e.g., 100°C or lower) that prevent this unnecessary oxidation or deterioration. This preserves the chemical identity of the support surface, which is often vital for the catalyst's interaction with reactants.
Mechanisms of Pore Structure Control
Leveraging Pressure Gradients
Solvent removal is not just about evaporation; it is about extraction from deep within the catalyst's porosity. A vacuum oven utilizes a pressure gradient to actively extract solution that has penetrated internal pores.
This mechanism ensures thorough removal of residual moisture and organic solvents. It maintains a loose powder structure, preventing the material from becoming dense or clumped, which facilitates subsequent processing steps like pyrolysis.
Preventing Surface Hardening
In standard atmospheric drying, liquid moves to the surface and evaporates, often leaving behind a solid "crust" that traps internal moisture—a phenomenon known as surface hardening.
The vacuum environment prevents this crust formation. By ensuring uniform evaporation driven by pressure rather than just surface temperature, it avoids trapping moisture and prevents the collapse of the material's morphology, ensuring the final powder remains porous.
Understanding the Trade-offs
Drying Rate Limitations
While effective, vacuum drying is generally slower than rapid convective or "quick drying" methods. It provides a methodical removal of solvents rather than an instantaneous flash-drying effect.
Impact on Metal Distribution Profile
The drying method influences where the metal settles within the support pellet. Vacuum drying typically results in an "egg-shell" layer thickness that is intermediate.
It produces a distribution that sits between the deep penetration of normal oven drying and the sharp exterior concentration of rapid drying. While the low-pressure environment reduces deep penetration to some extent, it may not achieve the extreme surface concentration of faster, higher-heat methods.
Making the Right Choice for Your Project
To maximize the efficacy of your platinum catalyst, select your drying method based on your specific stability and morphological requirements.
- If your primary focus is maximizing active surface area: Use vacuum drying to prevent particle agglomeration and preserve sub-nanometer dispersion.
- If your primary focus is structural integrity: Rely on the low-temperature vacuum environment to prevent pore collapse and the deactivation of surface functional groups.
- If your primary focus is manipulating shell thickness: Be aware that vacuum drying offers an intermediate distribution profile, distinct from the sharp shells produced by rapid convective drying.
By controlling pressure, you gain control over the microscopic architecture of your catalyst.
Summary Table:
| Feature | Vacuum Drying Impact | Technical Benefit |
|---|---|---|
| Temperature | Low-heat (e.g., 60-100°C) | Prevents thermal migration and sintering of Pt particles. |
| Pressure | Controlled Pressure Gradient | Extracts solvents from deep pores without structural collapse. |
| Particle Size | Sub-nanometer Preservation | Maintains high surface area and maximizes catalytic activity. |
| Morphology | Uniform Evaporation | Avoids surface hardening and ensures a loose, porous powder. |
Elevate Your Catalyst Synthesis with KINTEK
Precision is the hallmark of high-performance catalysts. KINTEK provides industry-leading vacuum drying ovens and high-temperature furnace systems designed to meet the rigorous demands of material science and R&D.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to protect your sub-nanometer active sites and ensure superior material morphology. Don't compromise your catalytic efficiency with suboptimal thermal processing.
Ready to optimize your lab's workflow? Contact us today to find your custom solution!
Visual Guide
References
- Hiroshi Yano. Sustainable activation of the PtCl <sub> <i>n</i> </sub> /Fe–N–C cathode for PEFCs through repeated subnanometer sizing and coarsening. DOI: 10.1039/d5lf00185d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What are the advantages of using an RTA system for CBTSe films? Precision Heating for Superior Thin Film Stoichiometry
- What is the function of placing a Nickel Mesh in a reactor? Maximizing Heat in Nickel-Hydrogen Systems
- What is the role of hydrate precursors in Mn3O4 nanosheet synthesis? Achieve Atomic-Level Dimensional Control
- What is the function of a high-temperature sintering furnace in ceramic membrane production? Engineered Performance
- How does a vacuum drying oven contribute to the quality of crosslinked precursors? Expert Guide to Material Integrity
- How does a laboratory oven function during PDMS curing? Achieve Precision in Device Encapsulation
- What is the function of a laboratory hot air drying oven in TiO2 treatment? Ensure Uniform Nanoparticle Quality
- What are advanced materials and composites? Unlock Superior Performance for Your Innovations



















