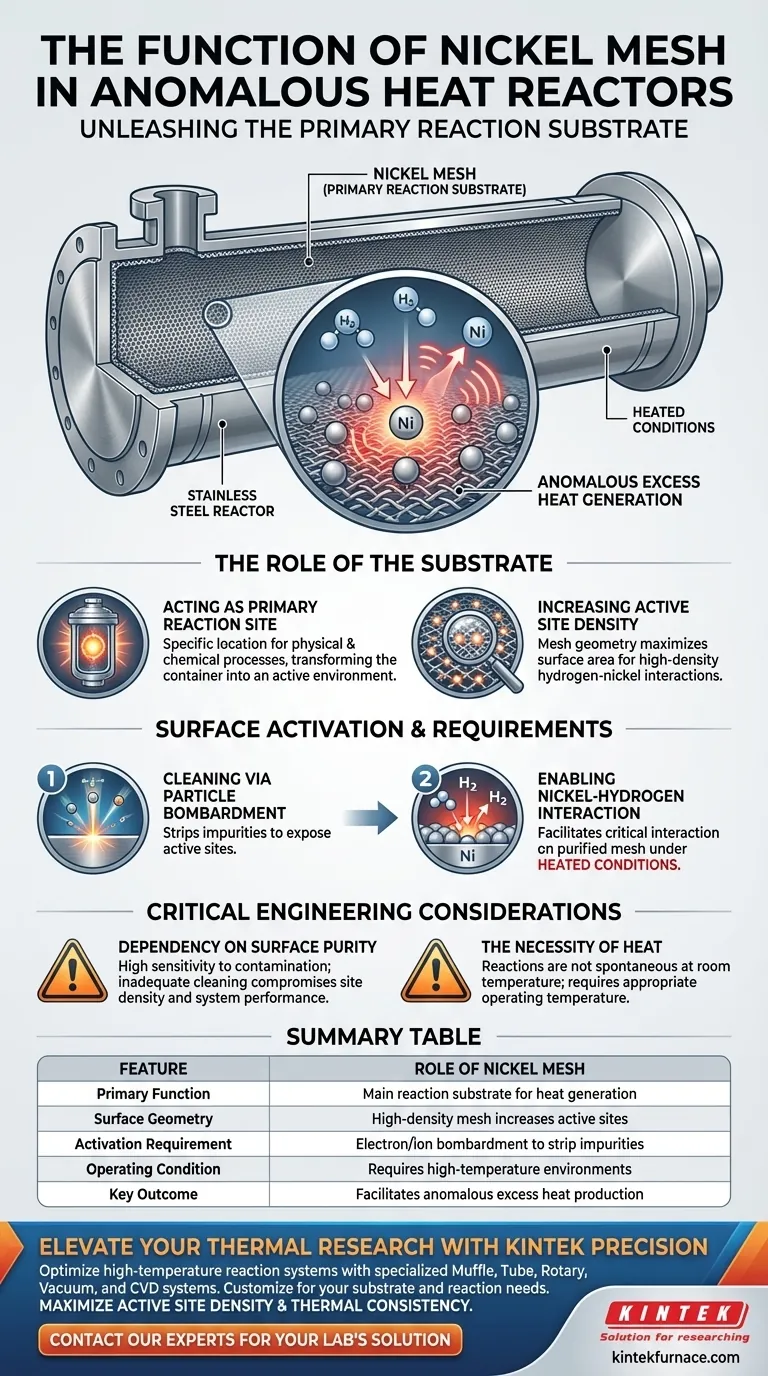
The Nickel Mesh functions as the primary reaction substrate within the anomalous heat generation system. Placed directly against the internal surface of the stainless steel reactor, it provides the necessary medium for nickel and hydrogen to interact under heated conditions, thereby driving the production of excess heat.
The mesh is not merely a structural component; it is the active engine of the system. By offering a high density of purified reaction sites, it maximizes the probability of the specific nickel-hydrogen interactions required to trigger anomalous thermal effects.

The Role of the Substrate
Acting as the Primary Reaction Site
The core function of the Nickel Mesh is to serve as the primary reaction substrate. It is the specific location where the physical and chemical processes driving the system occur.
By lining the internal wall of the Stainless Steel Reactor, the mesh transforms the vessel from a simple container into an active energetic environment.
Increasing Active Site Density
The geometry of a mesh is critical compared to a flat surface. This configuration is designed to provide a high density of active reaction sites.
A higher density of sites increases the surface area available for the hydrogen to interact with the nickel, directly influencing the system's potential to generate heat.
Surface Activation Requirements
Cleaning via Particle Bombardment
Simply placing nickel inside the reactor is insufficient; the surface condition is paramount. The mesh must be subjected to electron or ion bombardment.
This process is used to strip away impurities that naturally form on the metal. These impurities can block reaction sites and inhibit the system's performance.
Enabling Nickel-Hydrogen Interaction
Once the surface is purified, the mesh becomes highly reactive. Under heated conditions, this prepared surface facilitates the critical interaction between the nickel lattice and hydrogen.
It is this specific interaction on the clean mesh surface that promotes the generation of anomalous excess heat.
Critical Engineering Considerations
Dependency on Surface Purity
The reliance on electron or ion bombardment indicates a high sensitivity to contamination. If the mesh is not adequately cleaned, the density of active sites will be compromised, likely resulting in system failure.
The Necessity of Heat
The reaction is not spontaneous at room temperature. The reference explicitly states that these interactions occur under heated conditions, meaning the mesh functions only when the reactor is brought to the appropriate operating temperature.
Making the Right Choice for Your Goal
To maximize the efficacy of a nickel-based anomalous heat system, focus on the quality and preparation of the mesh interface.
- If your primary focus is Maximizing Heat Output: Prioritize the rigorous bombardment of the mesh to ensure the highest possible density of clean, active reaction sites.
- If your primary focus is System Consistency: Ensure the mesh is uniformly positioned against the stainless steel wall to maintain stable thermal conditions across the entire substrate.
The success of the reactor depends not just on the presence of nickel, but on the purity and activation of the mesh surface.
Summary Table:
| Feature | Role of Nickel Mesh in Reactors |
|---|---|
| Primary Function | Acts as the main reaction substrate for heat generation |
| Surface Geometry | High-density mesh increases active sites for hydrogen interaction |
| Activation Requirement | Must undergo electron/ion bombardment to strip impurities |
| Operating Condition | Requires high-temperature environments to trigger reactions |
| Key Outcome | Facilitates the production of anomalous excess heat |
Elevate Your Thermal Research with KINTEK Precision
Ready to optimize your high-temperature reaction systems? KINTEK provides the specialized equipment needed to drive consistent, high-output results. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces—all fully customizable to meet your unique substrate and reaction requirements.
Maximize your active site density and thermal consistency today. Contact our technical experts here to find the perfect solution for your lab's specific needs.
Visual Guide
References
- Tadahiko Mizuno, Jed Rothwell. Anomalous Heat Reaction from Hydrogen and Metals. DOI: 10.70923/001c.134027
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is electromagnetic induction heating considered environmentally friendly? Zero Emissions & High Efficiency
- Why is a forced-air drying oven necessary for impregnated kaolin catalysts? Achieve Uniform Component Immobilization
- Why is High-Temperature Annealing Required for WS2 Gas Sensors? Stabilize Performance & Eliminate Drift
- What critical environmental conditions does a high-temperature recrystallization annealing furnace provide? Maximize Steel Strength
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- What are the advantages of the sol-gel nitrate combustion method? Achieve Atomic-Level Purity in Oxide Synthesis
- What is the function of a high-temperature sintering furnace in ceramic membrane production? Engineered Performance
- Why is high temperature control precision essential for SiC/SiC composites? Master Microstructural Engineering



















