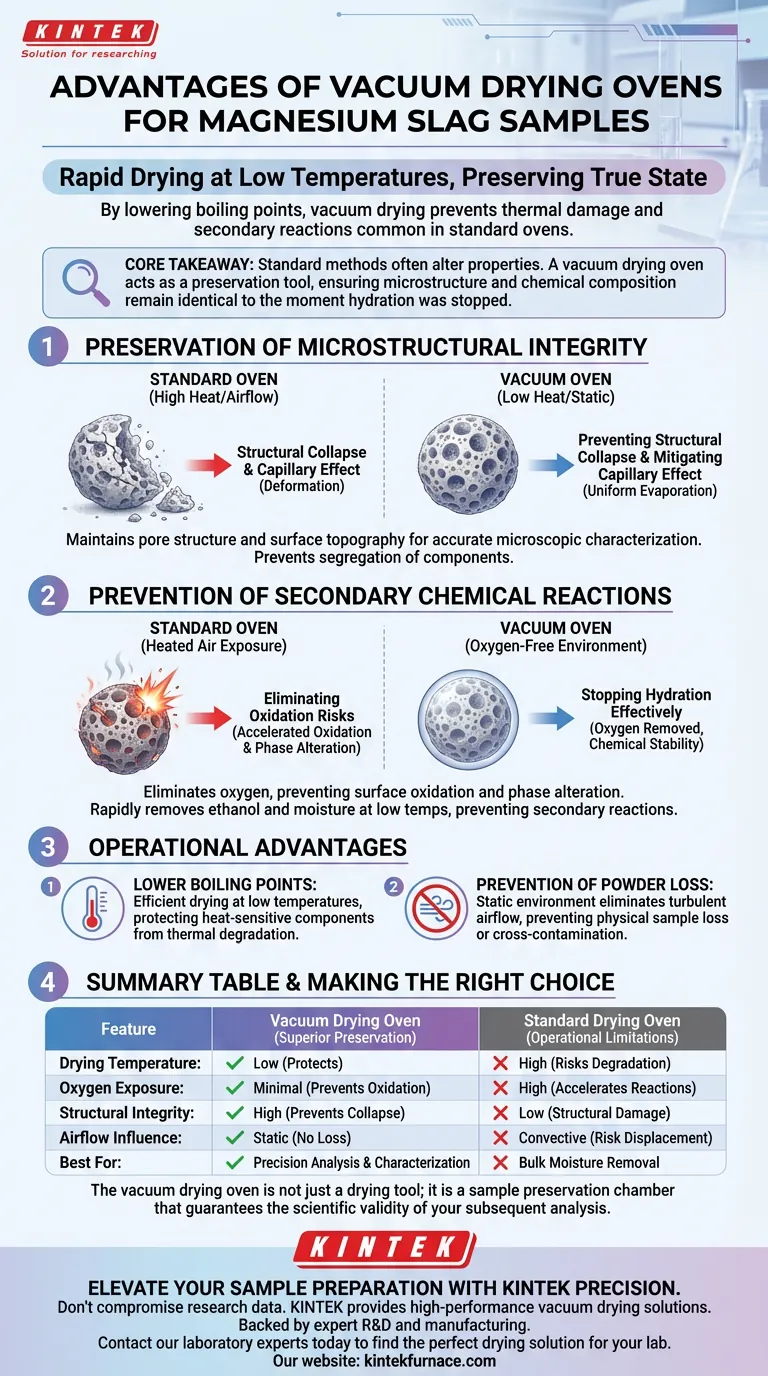
The primary advantage of using a vacuum drying oven for magnesium slag is its ability to dry samples rapidly at low temperatures, preserving their true physical and chemical state. By lowering the boiling point of solvents—such as the ethanol used to terminate hydration—vacuum drying prevents the thermal damage and secondary reactions common in standard high-temperature air ovens.
Core Takeaway Standard drying methods often alter the very properties you are trying to measure. A vacuum drying oven acts as a preservation tool, ensuring that the microstructure and chemical composition of the magnesium slag remain identical to their state at the moment hydration was stopped.

Preservation of Microstructural Integrity
The physical structure of magnesium slag is delicate, particularly after hydration. Standard drying equipment can destroy the features you need to analyze.
Preventing Structural Collapse
In a standard oven, high temperatures and air resistance can cause delicate microstructures to collapse or deform.
The vacuum environment allows moisture and solvents to evaporate quickly without the thermal stress associated with high heat. This ensures that the pore structure and surface topography of the slag are maintained, providing an accurate representation for microscopic characterization.
Mitigating the Capillary Effect
When drying occurs too rapidly at the surface (common in standard ovens), it creates a capillary effect. This pulls active components from deep pores toward the outer surface.
Vacuum drying promotes more uniform evaporation from within the material. This stability prevents the segregation of components and ensures that the internal distribution of elements remains consistent.
Prevention of Secondary Chemical Reactions
Magnesium slag is chemically reactive. The drying process must stop chemistry, not accelerate it.
Eliminating Oxidation Risks
Standard ovens expose samples to heated air, which accelerates oxidation. This can alter the chemical phase of the slag, leading to inaccurate data regarding its composition.
By removing air from the chamber, the vacuum oven eliminates the oxygen required for these reactions. This is critical for preventing surface oxidation and maintaining the chemical stability of the material.
Stopping Hydration Effectively
To study magnesium slag, researchers often use ethanol to terminate the hydration process.
A vacuum oven removes this residual ethanol and remaining moisture efficiently at low temperatures. This rapid removal prevents "secondary chemical reactions" that could occur if the solvents lingered or if the sample were exposed to high heat for prolonged periods.
Operational Advantages
Beyond sample integrity, the physics of vacuum drying offers practical benefits for handling powders and porous solids.
Lower Boiling Points
The vacuum environment significantly lowers the boiling point of liquids (like water and ethanol). This allows for thorough drying at temperatures that would normally be insufficient to drive off solvents, protecting heat-sensitive components from thermal degradation.
Prevention of Powder Loss
Standard ovens often rely on convection (airflow) to distribute heat. For fine powders like magnesium slag, this airflow can disturb the sample or blow powder away.
Vacuum drying operates in a static environment without turbulent airflow, eliminating the risk of physical sample loss or cross-contamination between samples.
Understanding the Trade-offs
While vacuum drying is superior for characterization, it is important to recognize the operational differences compared to standard equipment.
Throughput Limitations
Vacuum ovens typically have smaller chamber capacities than standard industrial drying ovens. They are designed for precision rather than high-volume bulk processing.
Maintenance Requirements
Maintaining a consistent vacuum requires vigilance regarding door seals and pump health. Unlike a standard oven, a vacuum leak can compromise the entire drying cycle.
Making the Right Choice for Your Goal
Deciding between a vacuum oven and a standard drying oven depends entirely on your analysis requirements.
- If your primary focus is microscopic characterization: You must use a vacuum drying oven to prevent structural collapse and ensure the features you see are authentic.
- If your primary focus is chemical phase analysis: You need a vacuum oven to prevent oxidation and secondary reactions that alter the sample's composition.
- If your primary focus is bulk moisture removal for non-critical applications: A standard oven may suffice, provided thermal degradation is not a concern.
The vacuum drying oven is not just a drying tool; it is a sample preservation chamber that guarantees the scientific validity of your subsequent analysis.
Summary Table:
| Feature | Vacuum Drying Oven | Standard Drying Oven |
|---|---|---|
| Drying Temperature | Low (protects heat-sensitive phases) | High (risks thermal degradation) |
| Oxygen Exposure | Minimal (prevents oxidation) | High (accelerates secondary reactions) |
| Structural Integrity | High (prevents pore collapse) | Low (capillary effect/structural damage) |
| Airflow Influence | Static (no powder loss) | Convective (risk of sample displacement) |
| Best For | Precision analysis & characterization | Bulk moisture removal (non-critical) |
Elevate Your Sample Preparation with KINTEK Precision
Don't compromise your research data with standard drying methods that alter your material's microstructure. KINTEK provides high-performance vacuum drying solutions designed to preserve the physical and chemical integrity of sensitive samples like magnesium slag.
Backed by expert R&D and manufacturing, KINTEK offers a wide range of laboratory equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research requirements.
Ready to ensure the scientific validity of your analysis? Contact our laboratory experts today to find the perfect drying solution for your lab.
Visual Guide
References
- Ping Lu, Xiaoming Liu. Structural Characteristics and Cementitious Behavior of Magnesium Slag in Comparison with Granulated Blast Furnace Slag. DOI: 10.3390/ma17020360
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What chemical role does phosphoric acid (H3PO4) play when activating biomass? Master Carbon Material Transformation
- Why do VTD sublimation capsules need specialized designs for perovskite? Achieve Precise Film Uniformity and Stability
- What are the structural advantages of specialized crystal growth furnaces for CZT? Achieve High-Purity Single Crystals
- How does an aluminum foil mask regulate temperature in the Floating-Zone process? Optimize Crystal Growth Precision
- What is the purpose of maintaining a 70°C environment in Li-NASICON experiments? Accelerate Your Battery Research
- What are the advantages of using a corundum crucible with a graphite sleeve in AlV55 alloy smelting? Ensure Pure Alloys
- Why is an in-situ XRD system with a high-temperature furnace necessary for Y-W-N ceramics? Capture Real-Time Stability
- How does Faraday's Law of Induction work in induction heating? Achieve Precise, Non-Contact Thermal Processing



















