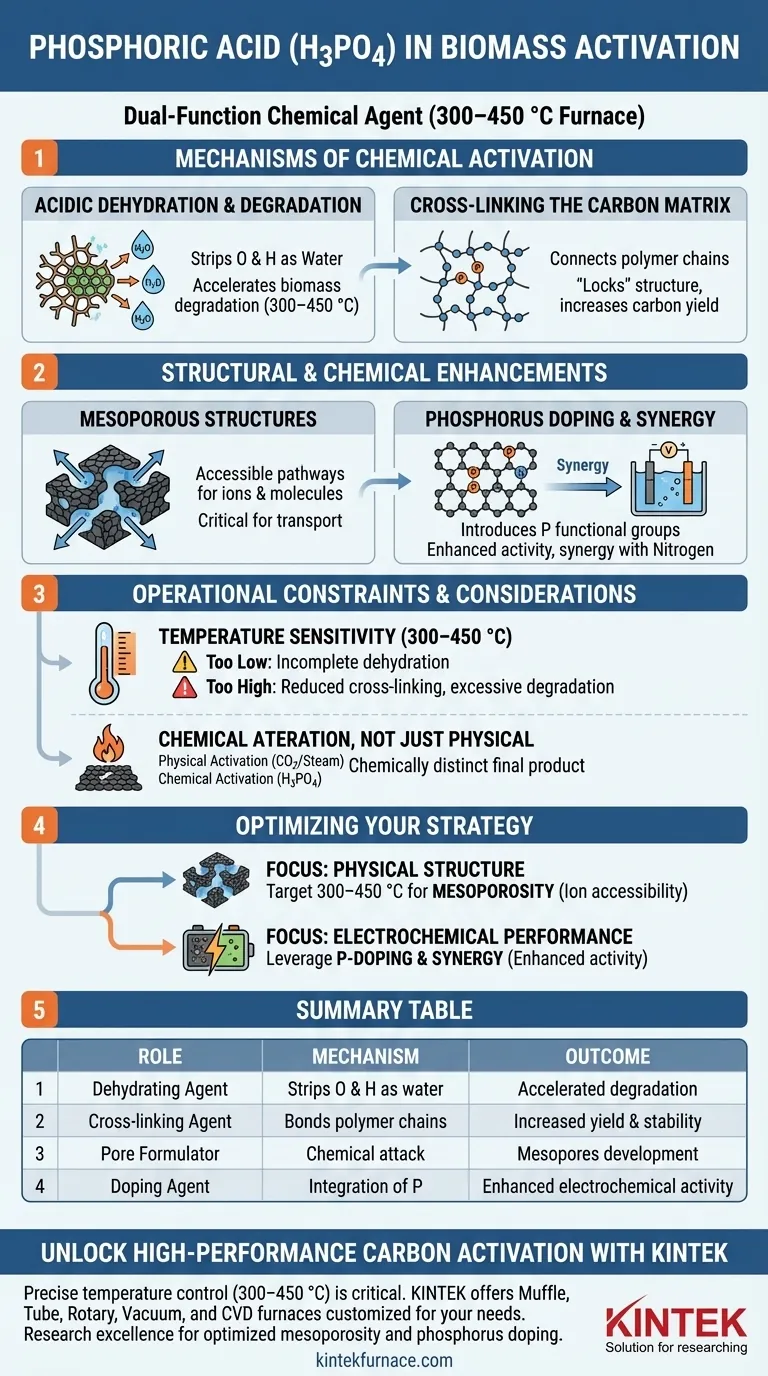
Phosphoric acid (H3PO4) serves as a dual-function chemical agent when activating biomass within a controlled temperature range of 300 to 450 °C. It acts simultaneously as a dehydrating agent and a cross-linking agent, accelerating the breakdown of biomass components while chemically restructuring the carbon framework.
Core Insight: H3PO4 does more than just create physical holes in the material; it fundamentally alters the surface chemistry. By introducing phosphorus functional groups and fostering mesoporosity, it creates a material specifically optimized for high-performance electrochemical applications.

Mechanisms of Chemical Activation
Acidic Dehydration and Degradation
At the molecular level, phosphoric acid acts as a potent dehydrating agent.
It aggressively strips oxygen and hydrogen (as water) from the biomass structure. This promotes the accelerated degradation of the biopolymer components (such as cellulose and lignin) at temperatures between 300 and 450 °C.
Cross-Linking the Carbon Matrix
Simultaneously, H3PO4 functions as a cross-linking agent.
It connects polymer chains within the biomass, essentially "locking" the carbon structure in place. This rigid framework prevents the excessive release of volatile organic matter, ensuring a higher yield of solid carbon.
Structural and Chemical Enhancements
Formation of Mesoporous Structures
The physical result of this chemical attack is the development of mesoporous structures.
Unlike micropores (which are very small), mesopores provide accessible pathways for ions and molecules. This architecture is critical for applications requiring rapid transport, such as in catalyst supports or electrode materials.
Phosphorus Doping and Synergy
The activation process inevitably leaves residual phosphorus bound to the carbon lattice.
This introduces phosphorus functional groups directly into the carbon matrix. When nitrogen is also present (nitrogen doping), these phosphorus groups create a synergistic effect that significantly enhances the material's electrochemical activity for energy storage and electrocatalysis.
Operational Constraints and Considerations
Temperature Sensitivity
The efficacy of H3PO4 is tightly bound to the thermal window of 300 to 450 °C.
Operating outside this specific range may alter the reaction pathway. If the temperature is too low, the dehydration may be incomplete; if too high, the cross-linking benefits may diminish or the carbon structure may degrade excessively.
Chemical Alteration vs. Physical Activation
You must recognize that this is a chemical modification, not just a physical one.
Unlike steam or CO2 activation, which primarily burn away carbon to create pores, H3PO4 chemically incorporates itself into the final product. This results in a material that is chemically distinct from the original precursor.
Optimizing Your Activation Strategy
To maximize the potential of your biomass-derived material, align your process parameters with your specific end-goal:
- If your primary focus is Physical Structure: Target the 300–450 °C window to maximize the formation of mesopores, ensuring ion accessibility for transport-heavy applications.
- If your primary focus is Electrochemical Performance: Leverage the H3PO4 treatment to introduce phosphorus functional groups, specifically looking for synergy with nitrogen doping to boost catalytic activity.
By strictly controlling the temperature and acid interaction, you transform waste biomass into a highly active, chemically tuned carbon material.
Summary Table:
| Activation Role | Chemical Mechanism | Physical & Chemical Outcome |
|---|---|---|
| Dehydrating Agent | Strips O and H as water at 300–450 °C | Accelerated degradation of cellulose/lignin |
| Cross-linking Agent | Bonds polymer chains into a rigid matrix | Increased carbon yield and structural stability |
| Pore Formulator | Chemical attack on biopolymers | Development of high-accessibility mesopores |
| Doping Agent | Integration of P into carbon lattice | Enhanced electrochemical activity (synergy with N) |
Unlock High-Performance Carbon Activation with KINTEK
Precise temperature control between 300°C and 450°C is critical for successful H3PO4 biomass activation. At KINTEK, we provide the expert R&D and manufacturing excellence needed to master this process.
Our range of Muffle, Tube, Rotary, Vacuum, and CVD systems are fully customizable to meet your unique chemical activation requirements. Whether you are optimizing mesoporosity for energy storage or enhancing electrochemical activity through phosphorus doping, our high-temperature furnaces ensure the thermal stability and precision your research demands.
Ready to elevate your material synthesis? Contact KINTEK today to find your perfect furnace solution!
Visual Guide
References
- Xing Huang, Dessie Ashagrie Tafere. Waste-derived green N-doped materials: mechanistic insights, synthesis, and comprehensive evaluation. DOI: 10.1039/d5su00555h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does high-temperature filtration equipment facilitate molten salt separation? Boost Your Slag Treatment Recovery
- What is the role of a laboratory drying oven in catalyst precursor control? Maximize Dispersion and Stability
- How do atomizers and furnaces function in Spray Pyrolysis? Master Nanoparticle Synthesis
- Why are high-precision constant temperature drying ovens required for potassium-sulfur batteries? Ensure Data Integrity
- What is the role of carbonaceous reducing agents in copper slag treatment? Maximize Metal Recovery with Expert Insights
- What are the core process advantages of using a microwave reactor? Maximize Speed & Efficiency in Lab Characterization
- How are the effects of heat treatment furnace parameters on AlSi10Mg evaluated? Master Microstructural Analysis
- How does a continuous argon flow heating chamber aid CMF testing? Ensure Pure Thermal Analysis



















