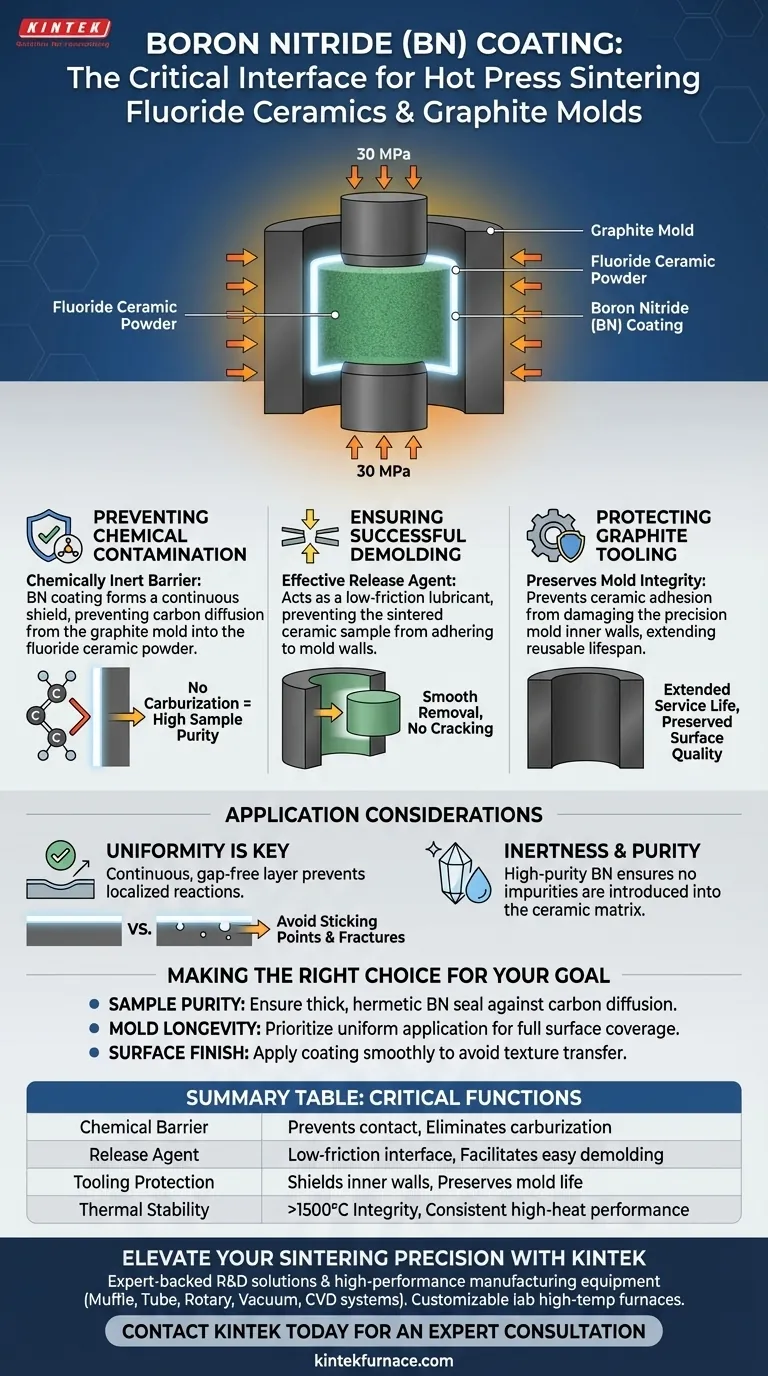
In the context of hot press sintering fluoride ceramics using graphite molds, a Boron Nitride (BN) coating functions primarily as a high-temperature isolation medium and release agent. It creates a physical and chemical barrier that prevents the reactive fluoride powder from interacting with the carbon-rich graphite mold.
Boron Nitride serves a dual purpose: it acts as a chemically inert shield to prevent carbon contamination (carburization) of the fluoride ceramic, and simultaneously functions as a lubricant to ensure the sintered sample does not fuse to the mold walls.

The Critical Functions of Boron Nitride
Hot press sintering involves subjecting materials to extreme heat and pressure. Without a protective interface, the graphite mold and fluoride powder would likely react or adhere to one another.
Preventing Chemical Contamination
At elevated sintering temperatures, graphite becomes chemically active.
Direct contact between the graphite mold and fluoride powder can lead to carburization, where carbon diffuses into the ceramic.
The Boron Nitride coating acts as a chemically inert barrier. This isolation prevents carbon impurities from degrading the purity and performance of the final fluoride ceramic.
Ensuring Successful Demolding
Under high pressure (often uniaxial pressure around 30 MPa) and high heat, ceramic powders tend to adhere to mold walls.
Boron Nitride acts as an effective release agent.
It prevents the sintered sample from sticking to the graphite, allowing for smooth removal without cracking the sample or damaging the mold.
Protecting the Graphite Tooling
Graphite molds are precision tools designed to maintain dimensional stability at temperatures often exceeding 1500°C.
Direct adhesion of the ceramic sample can damage the inner walls of the mold during removal.
By preventing this adhesion, the BN coating preserves the surface quality of the mold, extending its reusable lifespan.
Application Considerations
While Boron Nitride is essential, its effectiveness relies on proper application.
Uniformity is Key
The coating must be continuous. Any gap in the BN layer exposes the fluoride powder to the graphite.
Even small pinholes can lead to localized carburization or "sticking points" that may cause the sample to fracture during cooling or ejection.
Inertness and Purity
The Boron Nitride itself must be of high purity.
While it prevents carbon contamination, a low-quality coating could introduce its own impurities into the sensitive fluoride matrix.
Making the Right Choice for Your Goal
To maximize the success of your sintering run, prioritize the application of the coating based on your specific requirements:
- If your primary focus is Sample Purity: Ensure the BN layer is thick enough to create a complete hermetic seal against carbon diffusion.
- If your primary focus is Mold Longevity: Prioritize a uniform application that fully covers surface roughness to prevent mechanical interlocking and wear on the mold walls.
- If your primary focus is Surface Finish: Apply the coating smoothly to avoid transferring texture irregularities from the coating to the sintered ceramic surface.
A properly applied Boron Nitride coating is the single most effective way to safeguard both the chemical integrity of your fluoride ceramic and the mechanical integrity of your graphite tooling.
Summary Table:
| Function | Description | Benefit |
|---|---|---|
| Chemical Barrier | Prevents direct contact between graphite and fluoride powder | Eliminates carburization and ensures high sample purity |
| Release Agent | Provides a low-friction lubricating interface | Facilitates easy demolding without cracking samples |
| Tooling Protection | Shields the inner walls of the graphite mold | Preserves surface quality and extends mold service life |
| Thermal Stability | Maintains integrity at temperatures exceeding 1500°C | Ensures consistent performance during high-heat cycles |
Elevate Your Sintering Precision with KINTEK
Don't let carbon contamination or mold adhesion compromise your advanced material research. KINTEK provides expert-backed R&D solutions and high-performance manufacturing equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you are sintering sensitive fluoride ceramics or high-purity alloys, our lab high-temp furnaces are fully customizable to meet your unique thermal processing needs.
Ready to optimize your lab's efficiency and material integrity?
Contact KINTEK Today for a Expert Consultation
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What distinguishes a vacuum hot press furnace from simpler vacuum press systems? Unlock Advanced Material Densification
- What are the common applications of vacuum hot pressing? Essential for High-Performance Materials
- How does vacuum pressure control in an SPS furnace influence cemented carbide? Achieve High-Density Sintering Success
- How does an industrial-grade hot press sintering system benefit Al2O3/TiC/SiC(w) ceramics? Enhanced Material Density
- What industries benefit from the use of vacuum hot press furnaces? Unlock High-Performance Materials for Your Industry
- What is the function of a vacuum hot pressing furnace? Precision Diffusion Bonding for 321H Stainless Steel
- How does vacuum hot pressing equipment enhance the matrix quality of diamond tools through improved wettability? Unlock Superior Diamond Retention
- What is the primary role of mechanical pressure in Ti-Al vacuum hot pressing? Optimize Bonding and Density



















