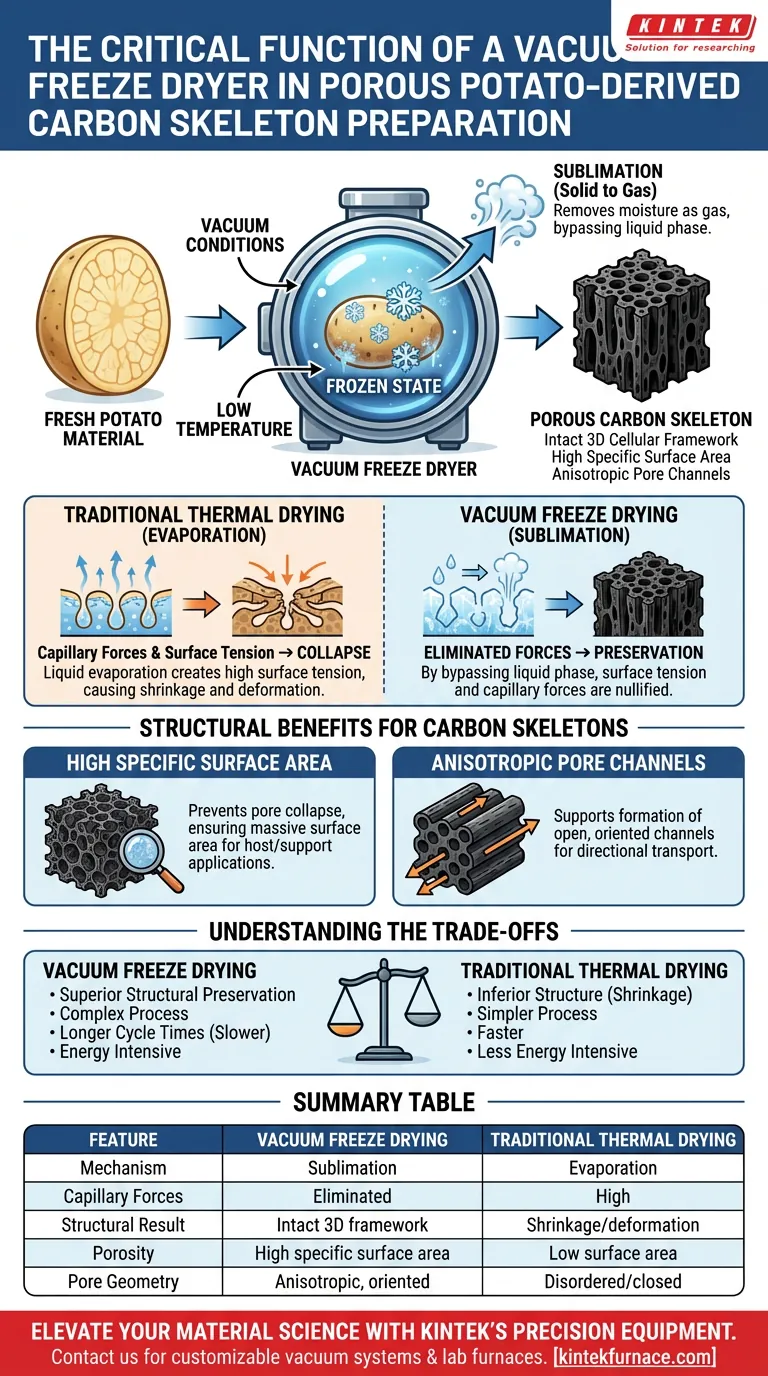
The critical function of a vacuum freeze dryer is to remove moisture from the potato material via sublimation, thereby preserving its intricate three-dimensional cellular structure. unlike traditional thermal drying, which often leads to shrinkage and structural collapse, freeze drying maintains the material's micro-morphology, creating a robust, porous carbon skeleton suitable for advanced applications.
By bypassing the liquid evaporation phase, vacuum freeze drying eliminates surface tension and capillary forces. This ensures the potato-derived carbon skeleton retains a high specific surface area and anisotropic pore channels, functioning as an effective support matrix for phase change materials.
The Mechanism of Preservation
Sublimation Over Evaporation
The defining characteristic of a vacuum freeze dryer is its ability to facilitate sublimation.
In this process, the water content within the potato is first frozen into a solid state. Under vacuum conditions, this ice converts directly into gas, bypassing the liquid phase entirely.
Eliminating Surface Tension
The primary danger during standard thermal drying is the creation of surface tension.
As liquid water evaporates from a porous material, surface tension creates powerful capillary forces. These forces pull the pore walls inward, causing the delicate biological framework to collapse or shrink.
Protecting the 3D Framework
Because freeze drying removes moisture as a gas rather than a liquid, capillary forces are effectively nullified.
This allows the potato's natural cellular structure to remain intact as the water is removed. The result is a rigid, dry skeleton that faithfully mirrors the volume and geometry of the original frozen material.
Structural Benefits for Carbon Skeletons
High Specific Surface Area
Preserving the micro-morphology directly translates to performance.
By preventing pore collapse, the freeze dryer ensures the final carbon material possesses a massive surface area. This property is essential when the skeleton is used as a host or support structure for other active materials.
Anisotropic Pore Channels
The freeze-drying process supports the formation of anisotropic (direction-dependent) structures.
The primary reference notes that this results in open, oriented pore channels. These channels are critical for applications requiring directional thermal conductivity or fluid transport within the carbon skeleton.
Controlling Pore Orientation
The freezing step preceding sublimation plays a vital role in structure definition.
By adjusting the freezing direction, one can induce the formation of specific, oriented pore architectures. This allows for the customization of the skeleton's internal geometry to meet specific engineering requirements.
Understanding the Trade-offs
Process Complexity
While superior in structural preservation, freeze drying is inherently more complex than thermal drying.
It requires precise control over both the freezing rate and the vacuum pressure. Failure to maintain the correct vacuum level can allow ice to melt back into liquid, reintroducing damaging capillary forces.
Time and Energy Intensity
Sublimation is a slower process than evaporation.
To achieve the desired structural integrity without damaging the micro-morphology, the cycle times are typically longer. This makes the process more resource-intensive compared to standard oven drying.
Making the Right Choice for Your Goal
When developing porous carbon materials from biomass, the drying method dictates the final architecture.
- If your primary focus is maximizing porosity: Use vacuum freeze drying to prevent capillary collapse and maximize specific surface area.
- If your primary focus is directional structure: Control the initial freezing direction before the vacuum stage to create oriented, anisotropic pore channels.
By utilizing sublimation, you transform a biological precursor into a high-performance engineering scaffold without compromising its structural fidelity.
Summary Table:
| Feature | Vacuum Freeze Drying | Traditional Thermal Drying |
|---|---|---|
| Mechanism | Sublimation (Solid to Gas) | Evaporation (Liquid to Gas) |
| Capillary Forces | Eliminated (No surface tension) | High (Causes structural collapse) |
| Structural Result | Intact 3D cellular framework | Shrinkage and pore deformation |
| Porosity | High specific surface area | Low surface area due to collapse |
| Pore Geometry | Anisotropic, oriented channels | Disordered and closed pores |
Elevate your material science research with KINTEK’s precision equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems and lab high-temp furnaces, including Muffle, Tube, Rotary, and CVD systems—all customizable for your unique biomass-derived carbon projects. Contact us today to optimize your lab's drying and carbonization processes!
Visual Guide
References
- Yuan Jia, Yushi Liu. Recent advances in energy storage and applications of form‐stable phase change materials with recyclable skeleton. DOI: 10.1002/cnl2.117
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Induction Melting Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is vacuum brazing considered a clean process? Achieve Oxide-Free, Flux-Free Metal Joining
- How does a vacuum distillation system achieve the separation of titanium? Advanced Metal Refining Insights
- What is vacuum brazing and how does it work? Achieve High-Strength, Clean Joints for Complex Assemblies
- What types of components are typically processed using vacuum carburizing? Boost Durability for Gears, Shafts, and More
- What are the advantages of using vacuum furnaces for sintering applications? Achieve Superior Metallurgical Control
- What are modified atmosphere furnaces and how do they differ from vacuum furnaces? Choose the Right Furnace for Your Process
- What safety benefits do vacuum furnaces offer? Inherently Safer High-Temperature Processing
- What are the advantages of using SSRs over contactors for heating control? Achieve Superior Precision in Vacuum Distillation



















