Vacuum distillation achieves separation by exploiting the differential volatility between titanium and liquid metal cathode materials. Specifically, the system relies on the significant gap in saturated vapor pressure between titanium and metals like tin or antimony to isolate the desired product.
The process subjects the metal mixture to high temperatures ($1500^\circ\text{C}$) and extremely low pressures ($1\text{Pa}$), causing the liquid cathode metals to evaporate while leaving high-purity titanium behind.
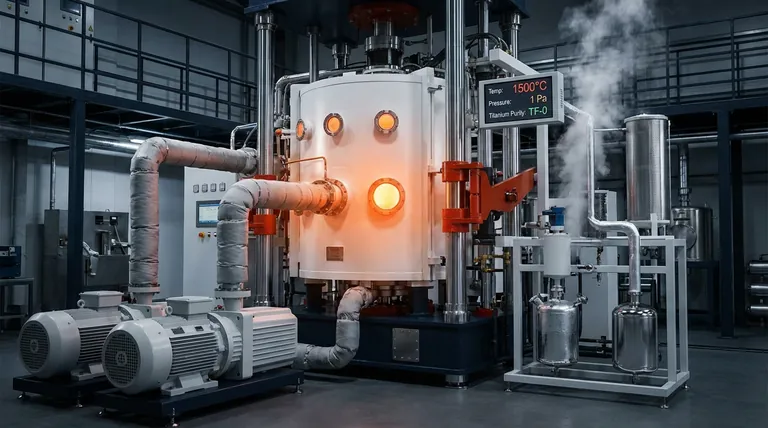
The Physical Mechanism of Separation
The Principle of Vapor Pressure
The core scientific principle driving this process is saturated vapor pressure.
Different metals transition from liquid to gas at vastly different rates under the same conditions. Titanium has a much lower vapor pressure compared to liquid cathode materials like tin or antimony.
Establishing the Environment
To trigger separation, the system creates an environment of extreme thermal energy and vacuum.
The process operates at approximately $1500^\circ\text{C}$. Simultaneously, the pressure is reduced to a near-vacuum state of roughly $1\text{Pa}$.
The Evaporation Phase
Under these specific conditions, the cathode metals (tin or antimony) reach their boiling points rapidly.
These metals vaporize, transitioning from the liquid phase into a gas. Because titanium has a lower vapor pressure, it remains stable in the container rather than evaporating.
Recovery and Condensation
The vaporized cathode metals are not lost; they are directed away from the heat source.
These vapors are subsequently condensed in a cooler section of the system. This allows for the recovery and potential reuse of the cathode materials.
The Resulting Purity
Isolation of Titanium
Once the volatile components have evaporated, the material remaining in the heating container is metallic titanium.
This residual titanium is solid or molten, depending on exact local conditions, but it is distinct from the evaporated impurities.
Meeting Industrial Standards
This method is highly effective for deep purification.
The process yields titanium capable of meeting rigorous industrial benchmarks, specifically TF-0 grade purity. This makes it suitable for high-performance applications.
Understanding the Operational Requirements
Energy Demands
Achieving separation requires significant energy input.
Maintaining a temperature of $1500^\circ\text{C}$ demands a robust power supply and specialized heating elements.
Vacuum Integrity
The efficiency of the separation is directly tied to the quality of the vacuum.
The system must reliably maintain $1\text{Pa}$ of pressure; any leaks or fluctuations will increase the boiling point of the impurities, stalling the separation process.
Optimizing for Purity and Recovery
If your primary focus is High-Grade Purity:
- Ensure the vacuum pressure is sustained at or below $1\text{Pa}$ to guarantee the complete removal of volatile impurities for TF-0 grade results.
If your primary focus is Material Recovery:
- Design the condensation zone to efficiently capture evaporated tin or antimony, minimizing waste of the cathode medium.
If your primary focus is Process Efficiency:
- Monitor the temperature strictly at $1500^\circ\text{C}$ to balance the speed of evaporation against energy consumption.
By precisely controlling the thermodynamic environment, you transform a complex mixture into high-value, purified titanium.
Summary Table:
| Parameter | Operating Condition | Outcome/Function |
|---|---|---|
| Temperature | $1500^\circ\text{C}$ | Drives evaporation of volatile cathode metals |
| Vacuum Pressure | $1\text{Pa}$ | Lowers boiling points to enable separation |
| Separation Basis | Vapor Pressure Gap | Titanium remains stable while tin/antimony vaporize |
| Product Quality | TF-0 Grade | High-purity metallic titanium suitable for industrial use |
| Secondary Phase | Condensation Zone | Recovery and reuse of liquid metal cathode materials |
Elevate Your Metal Refining with KINTEK Precision
Achieving TF-0 grade titanium requires absolute control over thermal and vacuum environments. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, CVD systems, and Muffle furnaces—all fully customizable to meet the rigorous $1500^\circ\text{C}$ and $1\text{Pa}$ demands of your lab or industrial refining process.
Ready to optimize your high-temperature applications? Contact us today to discover how our specialized heating solutions can enhance your material purity and process efficiency.
References
- C. X. Li, Yue Long. Advances in Integrated Extraction of Valuable Components from Ti-Bearing Slag. DOI: 10.3390/met15101080
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What critical process environments does a high-vacuum furnace provide for boron carbide? Achieve Superior Densification
- What role does a laboratory vacuum annealing furnace play in ion-implanted ScN thin films? Restore Lattice Integrity
- How are vacuum furnaces applied in the semiconductor industry? Essential for High-Purity Chip Manufacturing
- Why is dual monitoring used for Tantalum annealing? Achieve 20K Precision in Vacuum Furnaces
- How does a vacuum furnace prevent heat transfer and contamination? Achieve Ultimate Material Purity
- What temperature range can most vacuum furnace systems operate within? Discover the Capabilities for Your Process
- How does the atmosphere in a high vacuum sintering furnace affect 17-4PH properties? Optimize Density and Hardness
- What is the critical function of the water-cooled lid and body in a vacuum furnace during the magnesium distillation process?



















