The combination of a water-cooled copper crucible and repetitive flipping creates the optimal environment for synthesizing high-quality high-entropy alloys (HEAs). This approach simultaneously drives rapid solidification to refine the microstructure and utilizes mechanical agitation to guarantee the chemical uniformity required for these complex materials.
Core Takeaway Synthesizing high-entropy alloys requires precise control over both composition and structure. The water-cooled crucible ensures high purity and fine microstructure through rapid cooling and a "self-shielding" effect, while repetitive flipping eliminates macro-segregation to achieve critical chemical homogeneity.
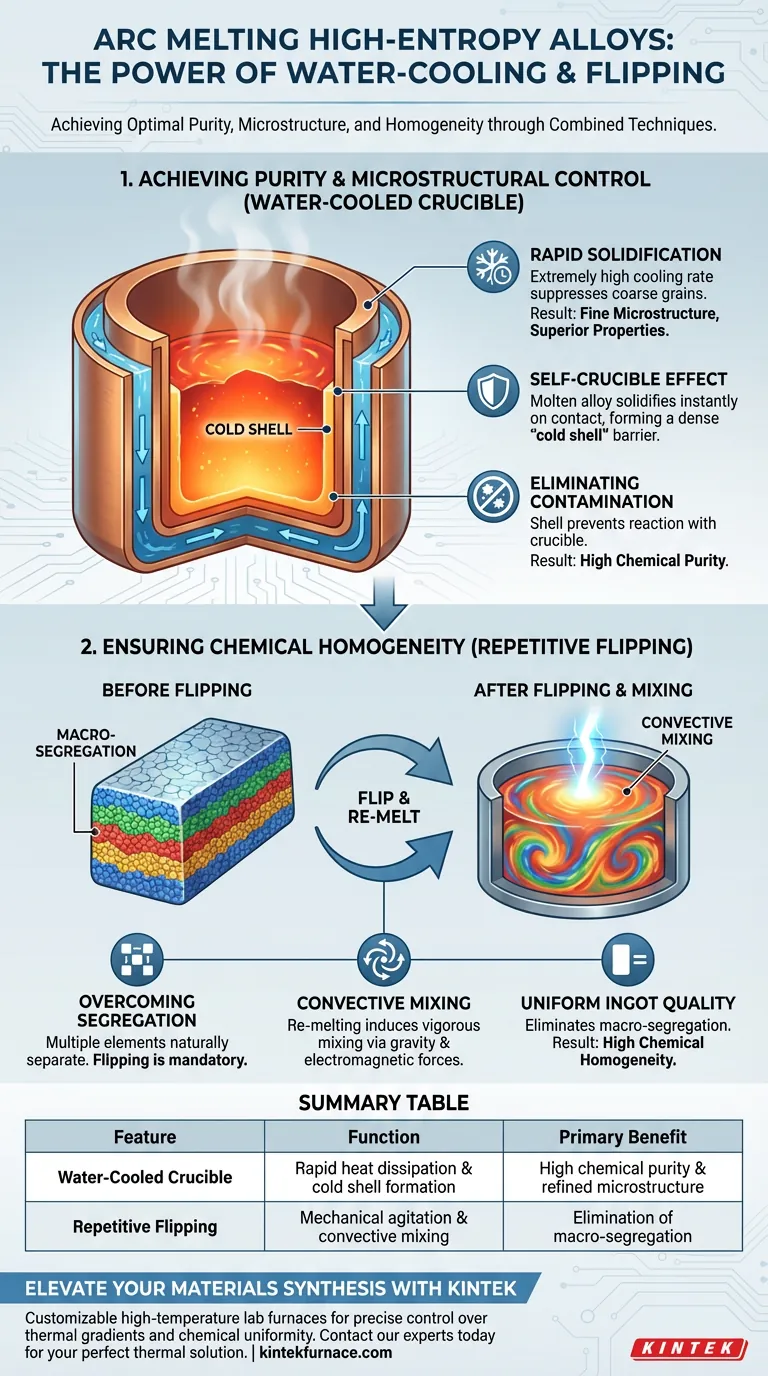
Achieving Purity and Microstructural Control
Rapid Solidification
The primary function of the water-cooled copper crucible is to facilitate an extremely high cooling rate during the melting process. This rapid heat extraction helps suppress the growth of large, coarse grains. Instead, it promotes the formation of fine solidified microstructures, which are generally associated with superior mechanical properties.
The Self-Crucible Effect
The circulating water rapidly dissipates heat, causing the molten alloy to solidify instantly upon contact with the crucible walls. This creates a dense, solid cold shell or condensation layer between the melt and the copper.
Eliminating Contamination
This solid shell acts as a barrier, preventing chemical reactions between the high-temperature melt and the crucible material. Unlike traditional ceramic crucibles, this "self-crucible" effect avoids the introduction of impurities, ensuring high chemical purity and precise composition of the alloy.
Ensuring Chemical Homogeneity via Flipping
Overcoming Segregation
High-entropy alloys are composed of multiple principal elements, making them naturally prone to macro-segregation. Without intervention, elements with different densities or melting points often separate, leading to inconsistent material properties.
Convective Mixing
Performing multiple flipping and re-melting operations is the mechanical solution to this problem. Each re-melt induces repeated convective mixing driven by gravity and the electromagnetic forces of the arc.
Uniform Ingot Quality
This rigorous agitation forces the various elements to blend thoroughly. The process effectively eliminates macro-segregation, resulting in an ingot with high chemical homogeneity throughout its entire volume.
Understanding the Constraints
The Necessity of Repetition
It is critical to understand that electric arc melting produces inherent compositional non-uniformity in a single pass. Flipping is not optional; it is a mandatory step to correct the segregation that naturally occurs during the initial melt.
Thermal Gradients
While the water-cooled crucible is excellent for purity, it creates a steep thermal gradient. The material touching the wall cools instantly, while the core remains molten longer. This can occasionally lead to minor microstructural variations from the surface to the center of the ingot.
Making the Right Choice for Your Goal
To maximize the quality of your high-entropy alloys, apply these principles based on your specific requirements:
- If your primary focus is Chemical Purity: Rely on the water-cooled copper crucible to create a "cold shell" that isolates your melt from external contaminants.
- If your primary focus is Material Consistency: Commit to multiple cycles of flipping and re-melting to ensure convective mixing fully eradicates macro-segregation.
By integrating rapid cooling with vigorous mechanical mixing, you ensure your alloy is both structurally refined and chemically chemically precise.
Summary Table:
| Feature | Function in Arc Melting | Primary Benefit |
|---|---|---|
| Water-Cooled Crucible | Rapid heat dissipation & cold shell formation | High chemical purity & refined microstructure |
| Repetitive Flipping | Mechanical agitation & convective mixing | Elimination of macro-segregation |
| Self-Crucible Effect | Solid shell barrier between melt and copper | Contamination-free processing |
| Rapid Solidification | Suppresses coarse grain growth | Superior mechanical properties |
Elevate Your Materials Synthesis with KINTEK
Precise control over thermal gradients and chemical uniformity is essential for high-performance High-Entropy Alloys. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized high-temperature lab furnaces—all fully customizable to meet your unique research needs.
Ensure the highest purity and homogeneity for your next breakthrough. Contact our experts today to find the perfect thermal solution for your laboratory.
Visual Guide
References
- Praise Mpofu, Lehlogonolo Rudolf Kanyane. RETRACTED: Mechanical and Tribological Performance of AlCrFeCuNi-(Vx) HEAs Synthesized via Arc Melting technique. DOI: 10.1051/e3sconf/202450501015
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Induction Melting Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the key steps in the vacuum induction melting process? Achieve High-Purity Metal Alloys for Demanding Applications
- Why is energy efficiency important when choosing an induction melting furnace? Cut Costs and Boost Productivity
- What is a Vacuum Induction Melting Furnace and where is it commonly used? Unlock High-Purity Alloy Production
- What are the key benefits of using induction furnaces for smelting precious metals? Maximize Purity and Yield
- How does an induction heating furnace compare to a resistance heating furnace in the production of ultrafine magnesium powder? Unlock 20X Higher Yield
- In which industries is vacuum melting technology commonly applied? Essential for Aerospace, Medical, and Electronics
- Why is it necessary to repeatedly flip and remelt ingots? Ensure Uniformity in Vacuum Arc Melting
- How does a high-frequency induction heating system contribute to the surface hardening of steel? Enhance Wear Resistance



















