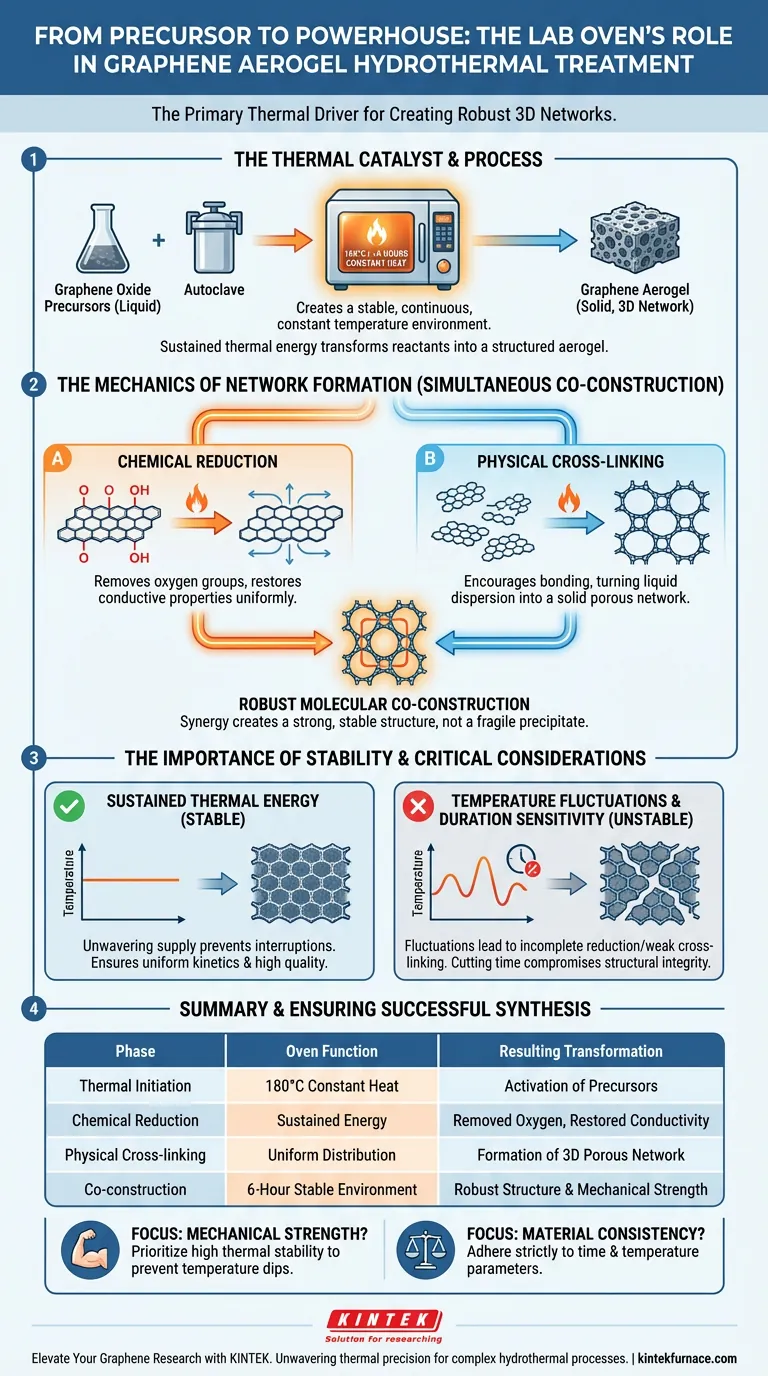
The laboratory oven functions as the primary thermal driver in the hydrothermal treatment of graphene aerogels. It creates a stable, continuous, and constant temperature environment—typically around 180°C for a duration of roughly 6 hours—which is applied to reactants sealed inside an autoclave. This sustained thermal energy is the essential catalyst that transforms graphene oxide precursors into a structured, three-dimensional aerogel.
The laboratory oven is not merely a heating unit; it is the guarantor of reaction stability. By maintaining precise thermal conditions, it ensures the simultaneous chemical reduction and physical cross-linking required to build a robust graphene network at the molecular level.

The Mechanics of Network Formation
The oven provides the energy required to fundamentally alter the chemical and physical state of the reactants. This process goes beyond simple drying; it acts as a synthesis reactor.
Driving Chemical Reduction
The heat supplied by the oven initiates the chemical reduction of graphene oxide. This removes oxygen-containing functional groups, restoring the conductive properties of the graphene. The oven ensures this reduction occurs uniformly throughout the sample.
Facilitating Physical Cross-Linking
Simultaneously, the thermal energy encourages physical cross-linking between graphene sheets. This cross-linking is what turns a liquid dispersion into a solid, porous network. The oven provides the activation energy needed for these sheets to bond effectively.
The Importance of Stability
The quality of the final aerogel depends heavily on the consistency of the environment provided by the oven.
Sustained Thermal Energy
The process requires a "continuous" input of heat over a set period, such as the 6-hour standard mentioned. The oven ensures that the energy supply does not waver, preventing interruptions in the reaction kinetics.
Robust Molecular Co-Construction
The combination of reduction and cross-linking is described as "co-construction." The oven's stable atmosphere allows these two processes to happen in tandem. This synergy results in a robust network structure rather than a fragile precipitate.
Critical Considerations and Trade-offs
While the oven is essential, understanding the limitations of thermal treatment is vital for consistent results.
Temperature Fluctuations
If the laboratory oven cannot maintain a strictly constant temperature, the network formation will be uneven. Fluctuations can lead to areas of incomplete reduction or weak cross-linking, compromising the mechanical strength of the aerogel.
Duration Sensitivity
The process relies on sustained energy input over time (e.g., 6 hours). Cutting this time short to save energy often results in a failure to achieve "robust co-construction." There is a direct trade-off between processing speed and the structural integrity of the graphene network.
Ensuring Successful Synthesis
To maximize the quality of your graphene aerogels, consider the following based on your specific objectives:
- If your primary focus is mechanical strength: Prioritize an oven with high thermal stability to ensure the "robust co-construction" of the network is not compromised by temperature dips.
- If your primary focus is material consistency: Adhere strictly to the required time and temperature parameters (e.g., 180°C for 6 hours) to guarantee uniform chemical reduction throughout the autoclave.
Ultimately, the laboratory oven provides the unwavering thermal foundation necessary to turn liquid precursors into high-performance solid materials.
Summary Table:
| Process Phase | Oven Function | Resulting Transformation |
|---|---|---|
| Thermal Initiation | Provides 180°C constant heat | Activation of precursors in autoclave |
| Chemical Reduction | Sustained energy input | Removal of oxygen groups; restored conductivity |
| Physical Cross-linking | Uniform thermal distribution | Formation of a 3D porous solid network |
| Co-construction | 6-hour stable environment | Robust molecular structure & mechanical strength |
Elevate Your Graphene Research with KINTEK
Achieving the perfect graphene aerogel requires more than just heat—it requires unwavering thermal precision. KINTEK provides industry-leading lab ovens, muffle furnaces, and vacuum systems designed to maintain the exact stability needed for complex hydrothermal processes.
Backed by expert R&D and manufacturing, our systems are fully customizable to your unique research demands. Ensure robust molecular co-construction and consistent material integrity with our high-temp solutions.
Ready to optimize your synthesis? Contact KINTEK today for a custom solution.
Visual Guide
References
- Martin Šilhavík, Jiří Červenka. Anderson Localization of Phonons in Thermally Superinsulating Graphene Aerogels with Metal‐Like Electrical Conductivity. DOI: 10.1002/smtd.202301536
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is a furnace with high-precision temperature control required for DPKB-S? Ensuring Material Synthesis Accuracy
- What are the specific temperature control requirements for alpha-SiC growth? Master the 1700°C Thermal Threshold
- What processes can continuous furnaces perform in a single step? Master Debinding and Sintering for High-Volume Production
- Why is the initial concentration of siloxane systems performed in a vacuum oven? Achieve Defect-Free Material Curing
- What role does a high-temperature sintering furnace play in lead-free piezoelectric ceramics? Optimizing Performance
- What role does a high-temperature blast drying oven play in nanocomposite formation? Ensure Structural Stability
- What are the advantages of supersonic inert gas cooling in DGCC? Transform Heat Treatment and Microstructure Control
- Why is a 1200°C hold required for Ti(C,N)-FeCr sintering? Unlock Superior Material Density



















