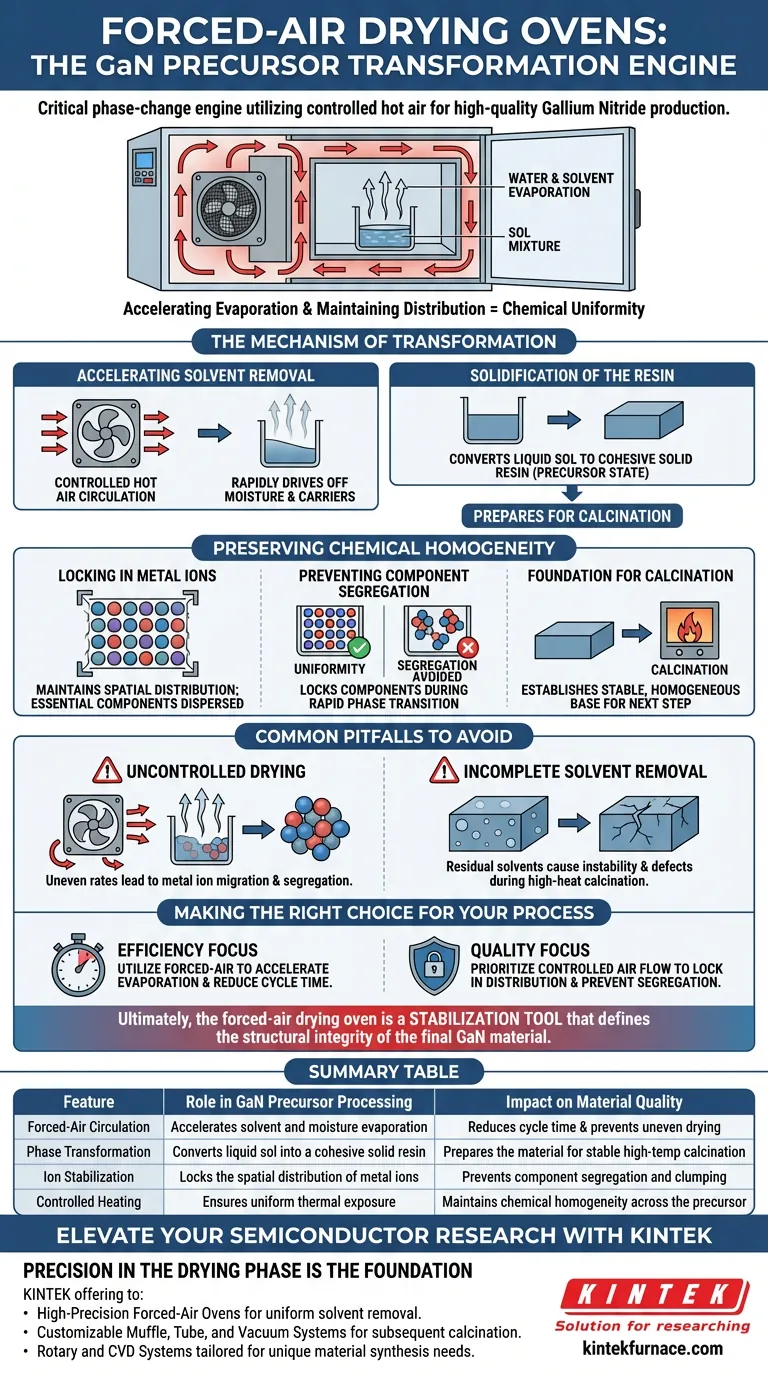
A forced-air drying oven serves as the critical phase-change engine in precursor processing. It utilizes controlled hot air circulation to accelerate the evaporation of water and solvents from the initial sol mixture, effectively converting the liquid solution into a solid resin.
By accelerating evaporation while maintaining the spatial distribution of metal ions, forced-air drying prevents component segregation and establishes the necessary chemical uniformity for high-quality Gallium Nitride production.
The Mechanism of Transformation
Accelerating Solvent Removal
The primary function of the oven is to drive off moisture and liquid carriers. By employing controlled hot air circulation, the system increases the rate of evaporation significantly compared to static drying methods.
This rapid removal of water and solvents is necessary to transition the material from a liquid "sol" state.
Solidification of the Resin
As the solvents evaporate, the physical state of the material changes. The forced-air process transforms the liquid sol into a cohesive solid resin.
This solid form is the required precursor state, readying the material for the high-temperature treatments that follow.
Preserving Chemical Homogeneity
Locking in Metal Ions
The most critical contribution of the forced-air drying oven is the preservation of internal structure. As the resin solidifies, the process maintains the specific spatial distribution of metal ions.
This ensures that the essential metallic components remain evenly dispersed throughout the material rather than clumping together.
Preventing Component Segregation
If drying occurs too slowly or unevenly, ingredients within a mixture can separate. The forced-air method specifically prevents this component segregation.
By locking the components in place during the rapid phase transition, the oven ensures the material remains chemically uniform.
Foundation for Calcination
This uniformity is not an end in itself; it is a prerequisite for the next step. The drying process establishes a stable foundation for subsequent calcination.
Without this stable and homogeneous resin, the final Gallium Nitride product would likely suffer from structural or chemical inconsistencies.
Common Pitfalls to Avoid
The Risk of Uncontrolled Drying
While forced-air drying is effective, the key operating principle is control. Reliance on passive drying or uneven air circulation can lead to inconsistent evaporation rates.
If the evaporation is not uniform, the metal ions may migrate, leading to the very segregation the process is designed to prevent.
Incomplete Solvent Removal
Failure to achieve a full transformation from sol to solid resin creates instability. Residual solvents trapped within the resin can cause defects during the high-heat calcination phase.
Ensuring the drying cycle is sufficient to fully solidify the resin is essential for downstream process integrity.
Making the Right Choice for Your Process
To maximize the quality of your Gallium Nitride precursors, ensure your drying protocol is aligned with your specific production goals.
- If your primary focus is process efficiency: Utilize forced-air circulation to significantly accelerate the evaporation of solvents and reduce total cycle time.
- If your primary focus is material quality: Prioritize controlled air flow to lock in metal ion distribution and prevent component segregation before calcination.
Ultimately, the forced-air drying oven is not just a heating element, but a stabilization tool that defines the structural integrity of the final GaN material.
Summary Table:
| Feature | Role in GaN Precursor Processing | Impact on Material Quality |
|---|---|---|
| Forced-Air Circulation | Accelerates solvent and moisture evaporation | Reduces cycle time and prevents uneven drying |
| Phase Transformation | Converts liquid sol into a cohesive solid resin | Prepares the material for stable high-temp calcination |
| Ion Stabilization | Locks the spatial distribution of metal ions | Prevents component segregation and clumping |
| Controlled Heating | Ensures uniform thermal exposure | Maintains chemical homogeneity across the precursor |
Elevate Your Semiconductor Research with KINTEK
Precision in the drying phase is the foundation of high-performance Gallium Nitride production. KINTEK provides the advanced thermal solutions necessary to lock in chemical uniformity and optimize your precursor transformation.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab equipment including:
- High-Precision Forced-Air Ovens for uniform solvent removal.
- Customizable Muffle, Tube, and Vacuum Systems for subsequent calcination.
- Rotary and CVD Systems tailored for unique material synthesis needs.
Don't let component segregation compromise your results. Contact KINTEK today to discover how our customizable high-temperature furnaces and drying solutions can enhance your lab's efficiency and material integrity.
Visual Guide
References
- Laser induced white emission and photocurrent of GaN nanoceramics. DOI: 10.1038/s41598-025-14109-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the primary function of a high-precision program-controlled furnace? Mastering T6 Heat Treatment of Al-Cu 224
- What is the technical necessity of sealing quartz ampoules at 10^-5 mbar for CVT? Ensure Crystal Purity
- Why is a high-pressure reactor with a PTFE lining required for V-NbOPO4@rGO? Ensure Purity in Acidic Synthesis
- What is the primary function of a laboratory drying oven in cotton stalk pyrolysis? Ensure Data Integrity
- How are thermal processing equipment commonly categorized? Choose the Right Furnace for Your Lab
- What morphological changes occur in POMOF after treatment? Unlock High Catalytic Performance via Thermal Evolution
- What is the purpose of preheating metal molds? Enhance Fluidity and Quality in Aluminum-Lithium Squeeze Casting
- How does a high-temperature laboratory furnace contribute to the formation of high-quality CsV3Sb5 single crystals?



















