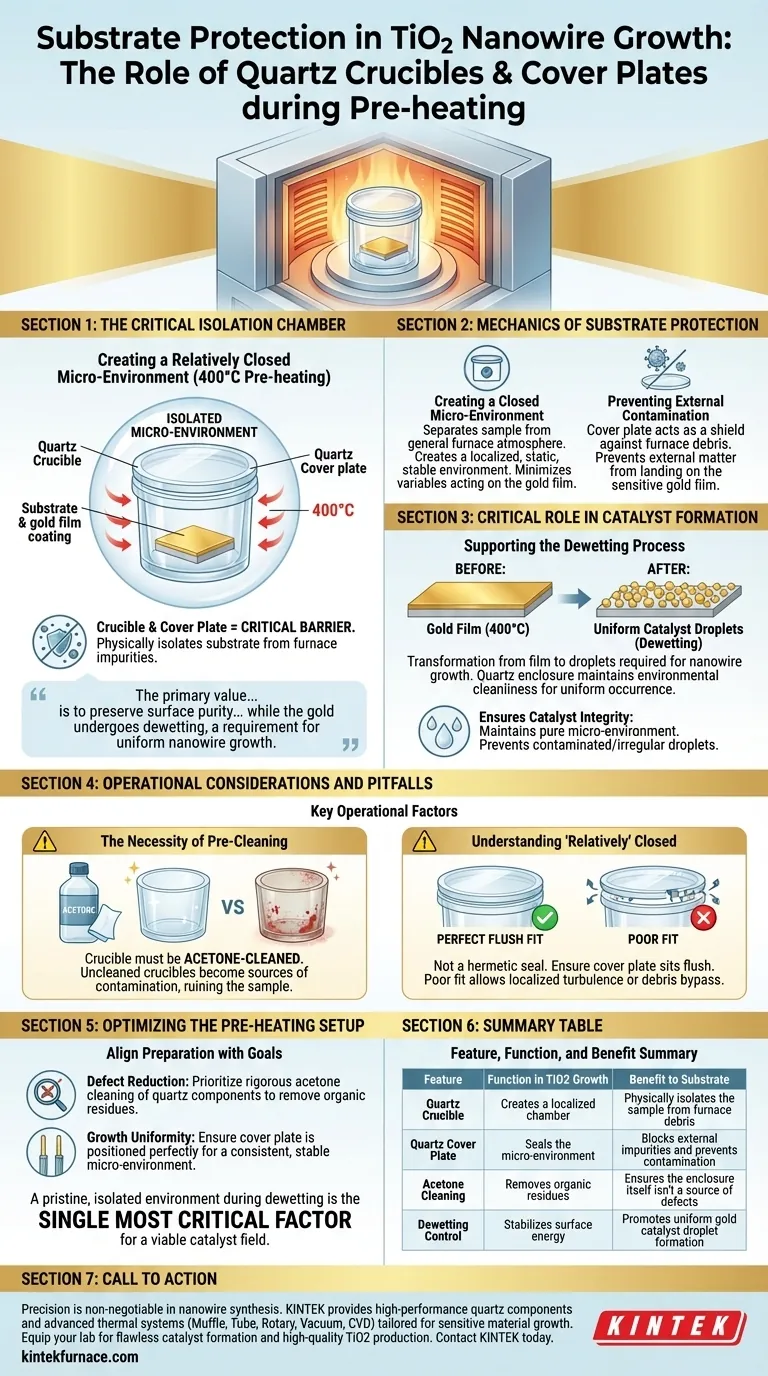
The combination of a quartz crucible and cover plate functions as a critical isolation chamber during the 400°C pre-heating phase. By sealing the gold-deposited substrate inside this assembly, you create a "relatively closed micro-environment" that physically blocks external impurities from contacting the sensitive gold film.
The primary value of the quartz assembly is to preserve surface purity during the transition from solid film to liquid catalyst. It ensures the environment remains contaminant-free while the gold undergoes dewetting, a requirement for uniform nanowire growth.

The Mechanics of Substrate Protection
Creating a Closed Micro-Environment
The fundamental role of the quartz crucible and cover plate is isolation. By placing the substrate inside and covering it, you effectively separate the sample from the general atmosphere of the furnace.
This configuration creates a localized, static environment. It minimizes the variables acting on the substrate, ensuring that the conditions immediately surrounding the gold film are controlled and stable.
Preventing External Contamination
Furnace environments can contain microscopic particulates or impurities. Without a physical barrier, these contaminants can settle on the substrate surface.
The quartz cover plate acts as a shield against this debris. It prevents external matter from physically landing on or interacting with the gold film during the sensitive heating ramp.
The Critical Role in Catalyst Formation
Supporting the Dewetting Process
At 400°C, the gold film on the substrate undergoes a process called dewetting. This is where the continuous film breaks apart to form the distinct catalyst droplets required for nanowire growth.
This transformation is highly sensitive to surface energy and chemistry. The quartz enclosure maintains the specific environmental cleanliness required for this physical change to occur uniformly.
Ensuring Catalyst Integrity
If impurities were to contact the gold film during dewetting, the resulting droplets could be contaminated or irregular. This would lead to poor nanowire growth or structural defects.
By maintaining a clean micro-environment, the quartz assembly ensures the gold remains pure. This allows the catalyst droplets to form correctly, setting the stage for high-quality nanowire synthesis.
Operational Considerations and Pitfalls
The Necessity of Pre-Cleaning
The protection offered by the quartz is only as good as the condition of the quartz itself. The primary reference explicitly notes that the crucible must be acetone-cleaned.
If the crucible is not rigorously cleaned before use, it becomes a source of contamination rather than a shield. Residue inside the "closed" environment will be trapped with the substrate, potentially ruining the sample.
Understanding "Relatively" Closed
The system creates a "relatively closed" environment, not a hermetic seal. While it blocks particulates, it allows for the necessary thermal equilibrium.
Operators should ensure the cover plate sits flush against the crucible. A poor fit compromises the micro-environment, allowing localized turbulence or external debris to bypass the protective barrier.
Optimizing the Pre-Heating Setup
To ensure the highest quality Titanium Dioxide nanowire growth, align your preparation with these specific goals:
- If your primary focus is Defect Reduction: Prioritize the rigorous acetone cleaning of the quartz crucible and cover plate to remove any organic residues before loading the substrate.
- If your primary focus is Growth Uniformity: Ensure the cover plate is positioned perfectly to create a consistent, stable micro-environment for the duration of the 400°C pre-heating phase.
A pristine, isolated environment during the dewetting phase is the single most critical factor for establishing a viable catalyst field.
Summary Table:
| Feature | Function in TiO2 Growth | Benefit to Substrate |
|---|---|---|
| Quartz Crucible | Creates a localized chamber | Physically isolates the sample from furnace debris |
| Quartz Cover Plate | Seals the micro-environment | Blocks external impurities and prevents contamination |
| Acetone Cleaning | Removes organic residues | Ensures the enclosure itself isn't a source of defects |
| Dewetting Control | Stabilizes surface energy | Promotes uniform gold catalyst droplet formation |
Precision is non-negotiable in nanowire synthesis. KINTEK provides high-performance quartz components and advanced thermal systems tailored for sensitive material growth. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique lab requirements. Contact KINTEK today to equip your lab with the tools needed for flawless catalyst formation and high-quality TiO2 production.
Visual Guide
References
- Zhina Razaghi, Guo‐zhen Zhu. Ni‐Assisted Endotaxial Growth of Au Nanoparticles Within TiO<sub>2</sub> Nanowires. DOI: 10.1002/admi.202500490
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is a vacuum drying oven necessary for Al2O3/TiC ceramic powders? Ensure Purity and Prevent Agglomeration
- Why are precision molds and laboratory presses critical for niobium-doped TiO2 ceramics? Achieve 94% Theoretical Density
- What is the function of a high alumina crucible in chloride salt purification? Protect Purity and Thermal Stability
- What functions does the hot pressing mold perform? Key Roles in Al3Ti/Al Composite Powder Metallurgy
- Why is a heat-resistant crucible indispensable for magnesium purification? Ensuring Purity and Efficiency in Vacuum Sublimation
- Why are long alumina boats selected as sample containers for zone refining experiments? Optimize Material Purification
- What role does a mass flow controller (MFC) play in gas distribution? Ensure Precision in Sensor Performance Evaluation
- How do alumina ceramic tubes compare to quartz ceramic tubes in terms of thermal properties? Choose the Right Tube for High-Temp Success



















