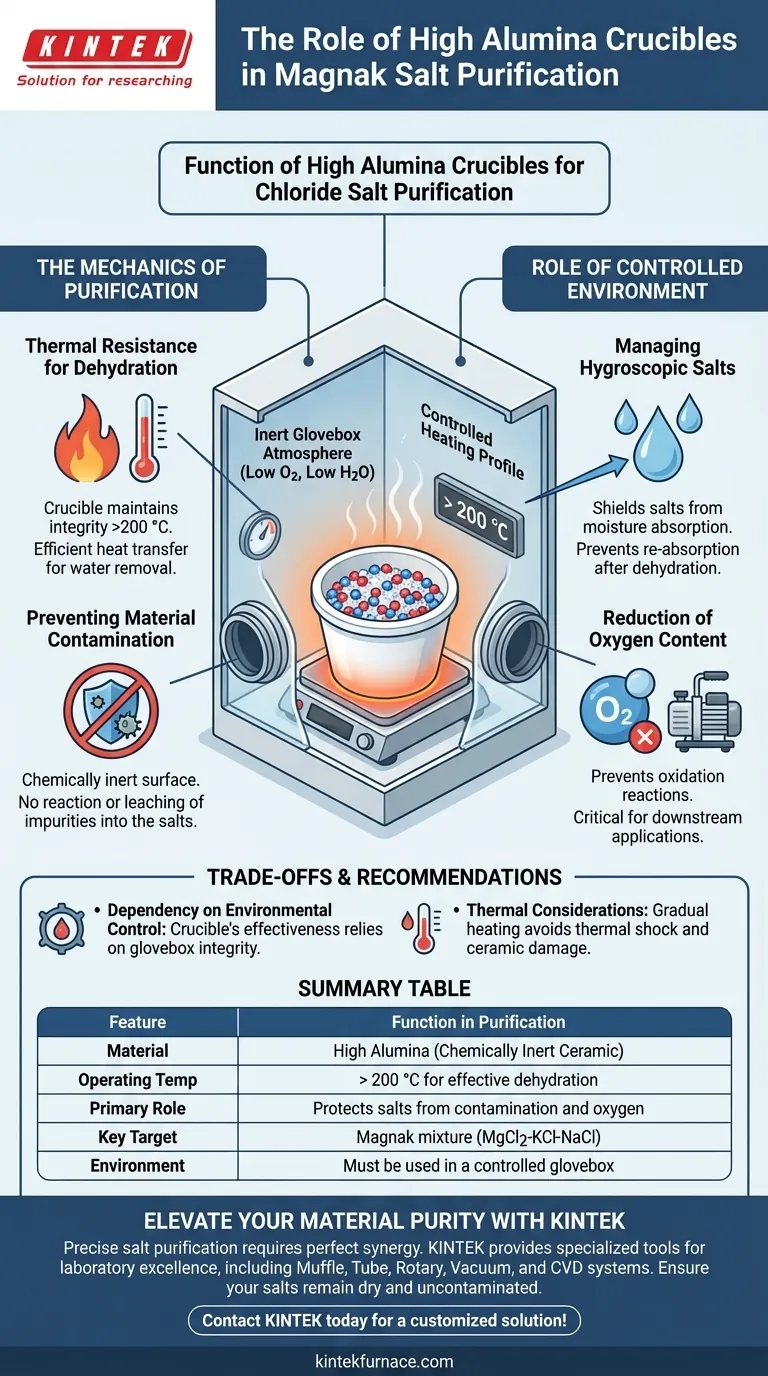
A high alumina crucible functions as a chemically inert, thermal-resistant vessel designed to protect hygroscopic chloride salts during the critical dehydration phase. Its primary role is to serve as a secure carrier within a controlled glovebox environment, allowing the Magnak mixture (MgCl2-KCl-NaCl) to be heated above 200 °C to remove moisture and oxygen without introducing impurities.
The high alumina crucible acts as a stabilizing barrier, enabling the necessary high heat for dehydration while isolating the reactive salts from the external atmosphere and containment contaminants.

The Mechanics of Purification
The choice of crucible material is not arbitrary; it is dictated by the chemical sensitivity of the salts and the thermal requirements of the process.
Thermal Resistance for Dehydration
To effectively purify the Magnak mixture, the salts must undergo dehydration at temperatures exceeding 200 °C.
A high alumina crucible is selected because it maintains structural integrity and chemical stability at these elevated temperatures. It acts as a reliable carrier that transfers heat efficiently to the salt mixture without degrading or softening.
Preventing Material Contamination
Chloride salts are highly susceptible to contamination during purification.
The high alumina composition provides a chemically inert surface that does not react with the salts or leach impurities into the mixture. This ensures that the raw material remains pure throughout the heating process.
The Role of the Controlled Environment
The crucible does not function in isolation; its utility is maximized by the environment in which it is used.
Managing Hygroscopic Salts
Salts like magnesium chloride (MgCl2) are hygroscopic, meaning they rapidly absorb moisture from the air.
The crucible is utilized within a laboratory glovebox to shield the salts from the external atmosphere. This containment system ensures that once moisture is driven off by the heat, it is not re-absorbed from the surrounding air.
Reduction of Oxygen Content
Beyond moisture, the purification process aims to minimize oxygen content within the salt mixture.
By holding the salts in a high alumina vessel inside an inert glovebox atmosphere, the system prevents oxidation reactions. This setup is critical for preparing the salts for downstream applications where oxygen impurities could be detrimental.
Understanding the Trade-offs
While high alumina crucibles are essential for this process, operators must understand the limitations of the setup to ensure success.
Dependency on Environmental Control
The crucible itself cannot prevent the re-absorption of moisture if the external environment is compromised.
The effectiveness of the high alumina vessel is entirely dependent on the integrity of the glovebox atmosphere. If the glovebox allows ambient air ingress, the inert properties of the crucible are rendered moot regarding moisture control.
Thermal Considerations
While alumina is heat resistant, it must be handled correctly to avoid thermal shock.
Rapid temperature changes can damage ceramic materials. Therefore, the heating profile within the glovebox must be controlled to reach >200 °C gradually to protect both the vessel and the integrity of the salt structure.
Making the Right Choice for Your Goal
To ensure the successful preliminary purification of Magnak mixtures, consider the following recommendations:
- If your primary focus is Purity: Rely on the high alumina crucible's inert properties to prevent leaching, but ensure the vessel is thoroughly cleaned before use.
- If your primary focus is Dehydration Efficiency: Verify that your glovebox heating element can consistently maintain the crucible temperature above 200 °C to drive off all bound moisture.
The high alumina crucible is the foundational tool that transforms raw, hygroscopic salts into a stable, dry precursor ready for advanced processing.
Summary Table:
| Feature | Function in Purification |
|---|---|
| Material | High Alumina (Chemically Inert Ceramic) |
| Operating Temp | > 200 °C for effective dehydration |
| Primary Role | Protects salts from contamination and oxygen |
| Key Target | Magnak mixture (MgCl2-KCl-NaCl) |
| Environment | Must be used in a controlled glovebox |
Elevate Your Material Purity with KINTEK
Precise salt purification requires the perfect synergy between high-performance vessels and controlled thermal environments. KINTEK provides the specialized tools you need to achieve laboratory excellence. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature lab furnaces designed to meet your unique purification requirements.
Ensure your hygroscopic salts remain dry and uncontaminated—Contact KINTEK today for a customized solution!
Visual Guide
References
- Mingyang Zhang, Jinsuo Zhang. Corrosion kinetics of pure metals (Fe, Cr, Ni) and alloys (A709, SS316) in thermal and chemical purified molten chloride salt. DOI: 10.1039/d5ra00451a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why are stainless steel tubes used during the cooling and heat treatment stages of Ti–Nb–Si alloys? Key Cooling Insights
- What are the risks of using high-purity alumina crucibles for periodate decomposition? Avoid Crucial Data Errors
- What is the role of mass flow controllers (MFC) in 2DP-F film preparation? Achieve High-Precision Synthesis Control
- What role does a Molybdenum Boat play in ZTO thin film deposition? Master Thermal Evaporation Success
- What should be evaluated when assessing supplier reliability for alumina ceramic furnace tubes? Ensure Consistent Performance and Support
- What are the preparation steps for a water circulating vacuum pump? Ensure Optimal Performance and Longevity
- What are the advantages of using aluminum crucibles for siloxane research? Maximize Thermal Precision and Data Accuracy
- What is the significance of using ceramic balls of varying diameters? Optimize Reactor Flow and Filtration



















