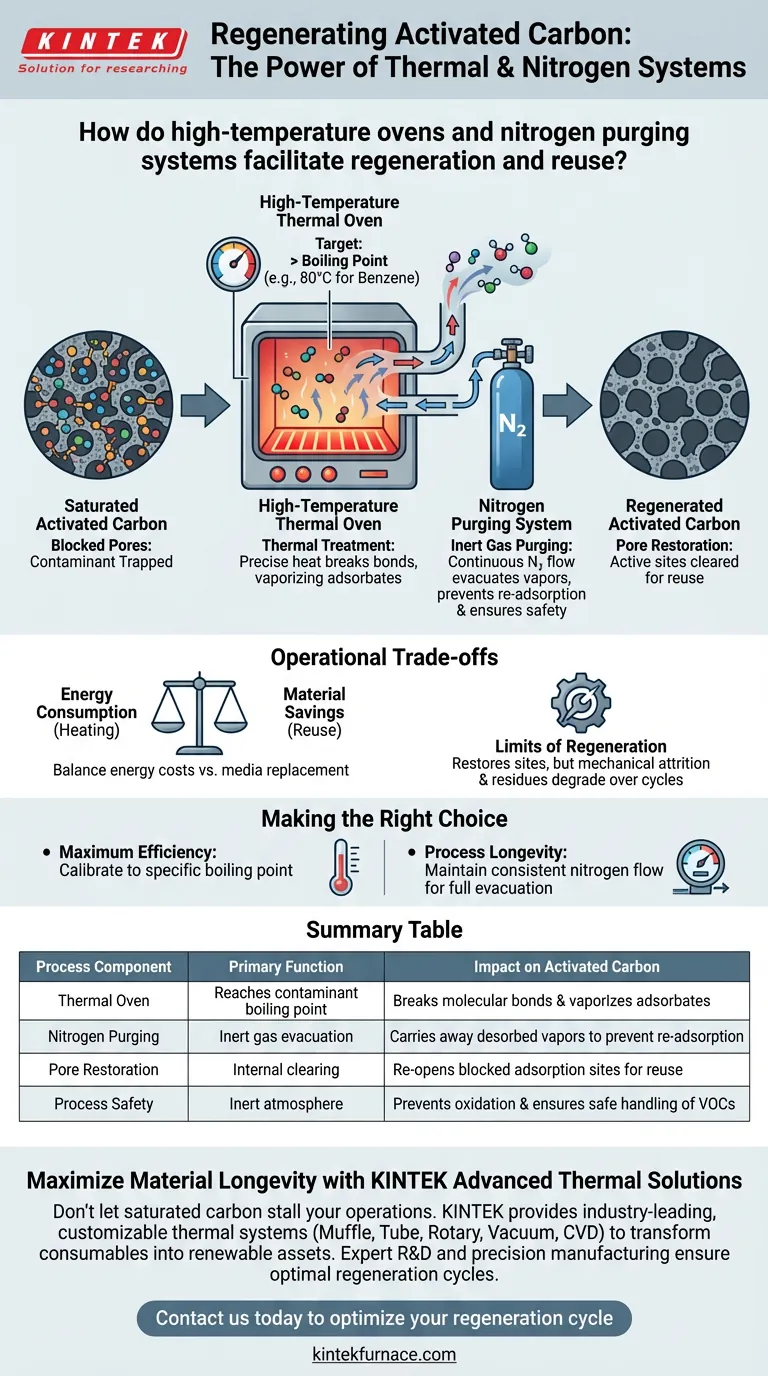
High-temperature thermal treatment combined with inert gas purging constitutes the primary mechanism for regenerating saturated activated carbon. The process involves using an oven to heat the carbon material to the specific boiling point of the adsorbed contaminants—such as 80 °C for benzene—causing them to vaporize, while a continuous stream of nitrogen gas physically sweeps these desorbed vapors away to prevent them from re-settling.
By applying precise heat to break the bond between the carbon and the contaminant, and utilizing nitrogen to evacuate the resulting vapors, you effectively clear blocked pores and restore active adsorption sites. This allows the material to maintain high efficiency across multiple usage cycles.

The Mechanics of Thermal Regeneration
Targeting the Boiling Point
The fundamental principle of regeneration is the application of heat to reverse the adsorption process.
The oven must raise the temperature of the saturated activated carbon to at least the boiling point of the adsorbed substance.
For example, if the carbon is saturated with benzene, the system must maintain a temperature of 80 °C. This thermal energy provides the necessary force to detach the contaminant molecules from the carbon's surface.
Restoring the Pore Structure
Activated carbon functions like a molecular sponge, trapping contaminants within its vast network of internal pores.
When these pores become blocked by captured substances, the carbon loses its effectiveness.
Thermal regeneration clears these blocked pores, effectively resetting the material's physical structure to a near-virgin state.
The Role of Nitrogen Purging
Evacuating Desorbed Vapors
Heat alone is insufficient for complete regeneration; it merely releases the contaminants from the carbon surface into the surrounding atmosphere within the oven.
Without a removal mechanism, these vapors would linger and potentially re-adsorb onto the carbon as it cools.
The nitrogen purging system acts as a carrier, continuously flowing through the chamber to transport the desorbed vapors out of the system.
Ensuring Process Safety and Efficiency
Nitrogen is used specifically because it is an inert gas.
It creates a controlled environment that facilitates the safe removal of volatile organic compounds without reacting with the carbon or the contaminants.
This ensures the process remains purely physical, focusing solely on the separation of the adsorbate from the adsorbent.
Operational Trade-offs
Energy Consumption vs. Material Savings
While regeneration saves the cost of purchasing new carbon, it introduces energy costs associated with heating the ovens.
You must balance the energy expenditure required to reach specific boiling points against the replacement cost of the filtration media.
Limits of Regeneration
Thermal regeneration is highly effective, but it does not make activated carbon last forever.
While the process restores adsorption sites, mechanical attrition and the accumulation of non-volatile residues may eventually degrade performance over many cycles.
Making the Right Choice for Your Goal
To maximize the value of your activated carbon system, consider the following regeneration strategies:
- If your primary focus is Maximum Efficiency: Ensure your oven temperature is precisely calibrated to the specific boiling point of the contaminant you are capturing.
- If your primary focus is Process Longevity: Maintain a consistent nitrogen flow rate to ensure all desorbed vapors are fully evacuated before the cooling phase begins.
Correctly implemented, this thermal-nitrogen cycle transforms activated carbon from a consumable supply into a renewable long-term asset.
Summary Table:
| Process Component | Primary Function | Impact on Activated Carbon |
|---|---|---|
| Thermal Oven | Reaches contaminant boiling point | Breaks molecular bonds and vaporizes adsorbates |
| Nitrogen Purging | Inert gas evacuation | Carries away desorbed vapors to prevent re-adsorption |
| Pore Restoration | Internal clearing | Re-opens blocked adsorption sites for reuse |
| Process Safety | Inert atmosphere | Prevents oxidation and ensures safe handling of VOCs |
Maximize Material Longevity with KINTEK Advanced Thermal Solutions
Don't let saturated carbon stall your operations. KINTEK provides industry-leading, customizable thermal systems designed to transform your consumables into renewable assets. Backed by expert R&D and precision manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your specific regeneration temperatures.
Our lab high-temperature furnaces ensure precise calibration and uniform nitrogen flow, protecting your pore structures and maximizing adsorption efficiency. Contact us today to optimize your regeneration cycle and discover how our specialized engineering can reduce your long-term material costs.
Visual Guide
References
- Sinan Kutluay, Orhan Baytar. Enhanced benzene vapor adsorption through microwave-assisted fabrication of activated carbon from peanut shells using ZnCl2 as an activating agent. DOI: 10.1007/s11356-024-32973-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- For which materials is the experimental box type atmosphere furnace suitable? Ideal for Metals, Ceramics, and Advanced Materials
- What is the primary purpose of using a constant temperature drying oven in sugarcane bagasse pretreatment? Find Out Why
- What is the significance of atmosphere furnaces in the ceramics and glass industry? Unlock Precision in High-Temperature Processing
- What are the purposes of inert atmospheres in heat treatment? Enhance Metal Quality and Safety
- How does a horizontal box furnace facilitate atmosphere control in the synthesis of Ni-TiON catalysts?
- What is the function of an industrial resistance furnace in melting Al-Fe-Ni-Sc-Zr alloys? Achieve Alloy Homogeneity
- Why is a tube atmosphere furnace required for sulfur-doped hard carbon? Master Precision Carbon Synthesis
- What are the advantages of using a controlled atmosphere furnace? Achieve Precise Material Processing and Quality



















