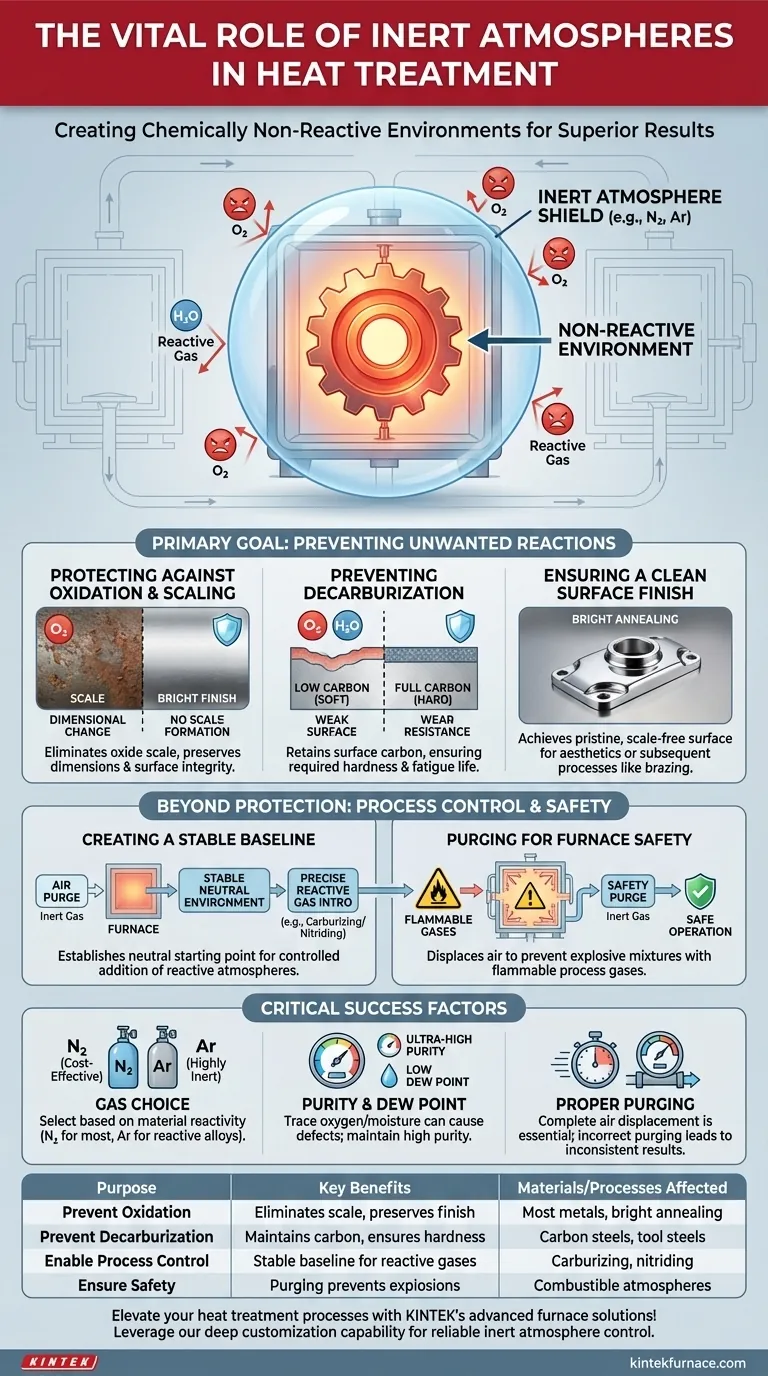
At its core, an inert atmosphere serves one primary purpose in heat treatment: to create a chemically non-reactive environment. This controlled atmosphere shields heated metal components from reacting with the air, primarily oxygen, preventing detrimental effects like oxidation and ensuring the final part meets its required quality and performance specifications.
The use of an inert atmosphere is not merely a protective measure; it is a fundamental tool for process control. It guarantees that the only changes occurring in the material are those intended by the heat treatment cycle itself, while also ensuring the operational safety of the furnace.

The Primary Goal: Preventing Unwanted Reactions
When metals are heated, their reactivity increases dramatically. An inert atmosphere, typically composed of nitrogen or argon, displaces the reactive gases found in ambient air to preserve the material's integrity.
Protecting Against Oxidation and Scaling
At high temperatures, oxygen in the air readily reacts with most metals to form a layer of oxide, commonly known as scale. This scale alters the part's dimensions, ruins its surface finish, and can interfere with subsequent processes like brazing or plating.
An inert gas environment eliminates the available oxygen, completely preventing scale formation.
Preventing Decarburization
For carbon steels, the oxygen and water vapor in air can react with the carbon near the metal's surface. This process, called decarburization, depletes carbon from the surface layer.
Since carbon is the primary hardening element in steel, decarburization results in a soft, weak surface, compromising the part's wear resistance and fatigue life. An inert atmosphere protects the surface carbon content.
Ensuring a Clean Surface Finish
Processes like bright annealing are designed to soften a metal without altering its surface appearance. By preventing any oxidation, an inert atmosphere ensures the part emerges from the furnace with a clean, bright, and scale-free finish.
This is critical for parts where aesthetics are important or where a pristine surface is needed for a subsequent joining process like brazing.
Beyond Protection: Enabling Process Control and Safety
While preventing unwanted reactions is the main goal, inert gases also play a critical role in establishing a baseline for more complex processes and ensuring the furnace operates safely.
Creating a Stable, Controlled Environment
Many heat treatment processes, such as carburizing or nitriding, intentionally add reactive gases to the furnace to alter the surface chemistry of a part.
In these cases, an inert gas is first used to purge all air from the furnace. This creates a neutral, predictable starting point, allowing for the precise and repeatable introduction of a controlled amount of reactive gas.
Purging for Furnace Safety
Heat treating furnaces often use flammable or combustible atmospheres (e.g., endothermic gas, ammonia). Introducing these gases into a furnace containing air (oxygen) at high temperatures can create an explosive mixture.
Inert gases are used as a safety purge. The furnace is first filled with nitrogen or argon to displace all oxygen before the flammable process gas is introduced. The same purge is performed at the end of the cycle to remove the flammable gas safely.
Understanding the Trade-offs and Considerations
Simply choosing to use an inert gas is not enough. The specific gas, its purity, and the furnace's integrity are all critical factors for success.
Choosing the Right Gas: Nitrogen vs. Argon
Nitrogen (N2) is the most common and cost-effective inert gas. However, at very high temperatures, it can react with certain alloys like titanium, stainless steels, and some tool steels to form undesirable nitrides.
Argon (Ar) is more truly inert than nitrogen and is used for highly reactive materials or at higher process temperatures where nitrogen reactivity is a concern. Its primary drawback is its significantly higher cost.
The Importance of Purity and Dew Point
The effectiveness of an inert atmosphere depends on its purity. Even trace amounts of oxygen or moisture (measured as dew point) in the gas supply can be enough to cause surface oxidation or decarburization on sensitive materials.
Ensuring a high-purity gas supply and leak-tight furnace integrity is essential for achieving optimal results.
The Risk of Incomplete Purging
Failing to completely purge the air from a furnace before heating is a common mistake. If pockets of air remain, it will lead to inconsistent, spotty oxidation on the parts, often resulting in rejection and rework. Proper purge times and flow rates are critical process parameters.
Making the Right Choice for Your Process
The decision to use an inert atmosphere is driven by the desired outcome for your component. Your primary goal will dictate the level of control required.
- If your primary focus is surface quality and appearance: An inert atmosphere is non-negotiable to prevent oxidation and achieve a bright, clean finish for processes like annealing or brazing.
- If your primary focus is mechanical performance: Preventing decarburization is critical, as it directly preserves the hardness and fatigue strength of the component's surface.
- If your primary focus is process safety and control: Using an inert gas purge is a fundamental step to safely introduce or remove reactive or flammable atmospheres in processes like carburizing.
Mastering atmosphere control is a cornerstone of modern, high-quality heat treatment.
Summary Table:
| Purpose | Key Benefits | Materials/Processes Affected |
|---|---|---|
| Prevent Oxidation | Eliminates scale formation, preserves surface finish | Most metals, bright annealing |
| Prevent Decarburization | Maintains surface carbon, ensures hardness | Carbon steels, tool steels |
| Enable Process Control | Provides stable baseline for reactive gases | Carburizing, nitriding |
| Ensure Safety | Purging flammable gases to prevent explosions | Furnaces with combustible atmospheres |
Elevate your heat treatment processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, helping you achieve superior material quality and operational safety. Contact us today to discuss how we can optimize your heat treatment with reliable inert atmosphere control!
Visual Guide
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the advantage of using nitrogen as a filling gas? Ensure High Yield Silica Extraction from Biomass
- What are the two main purposes of controlling the atmosphere in a lab furnace? Master Material Protection and Transformation
- What role does an industrial oven play in the pre-treatment of Licuri bark? Optimize Activated Carbon Production
- What energy-saving and environmental benefits do box type atmosphere furnaces provide? Boost Efficiency and Cut Waste
- What is the purpose of pre-heating industrial-grade ceramic molds? Ensure Perfect Grain Structures and Casting Yield
- What are the applications of an atmosphere box furnace in environmental protection? Safely Treat Hazardous Waste with Precision
- Why is an inert atmosphere required for Mo6S8 annealing at 1000°C? Ensure High-Purity Cathode Synthesis
- How does an oxygen atmosphere furnace help optimize the optical performance of SiO2 microarchitectures? Enhancing Clarity



















