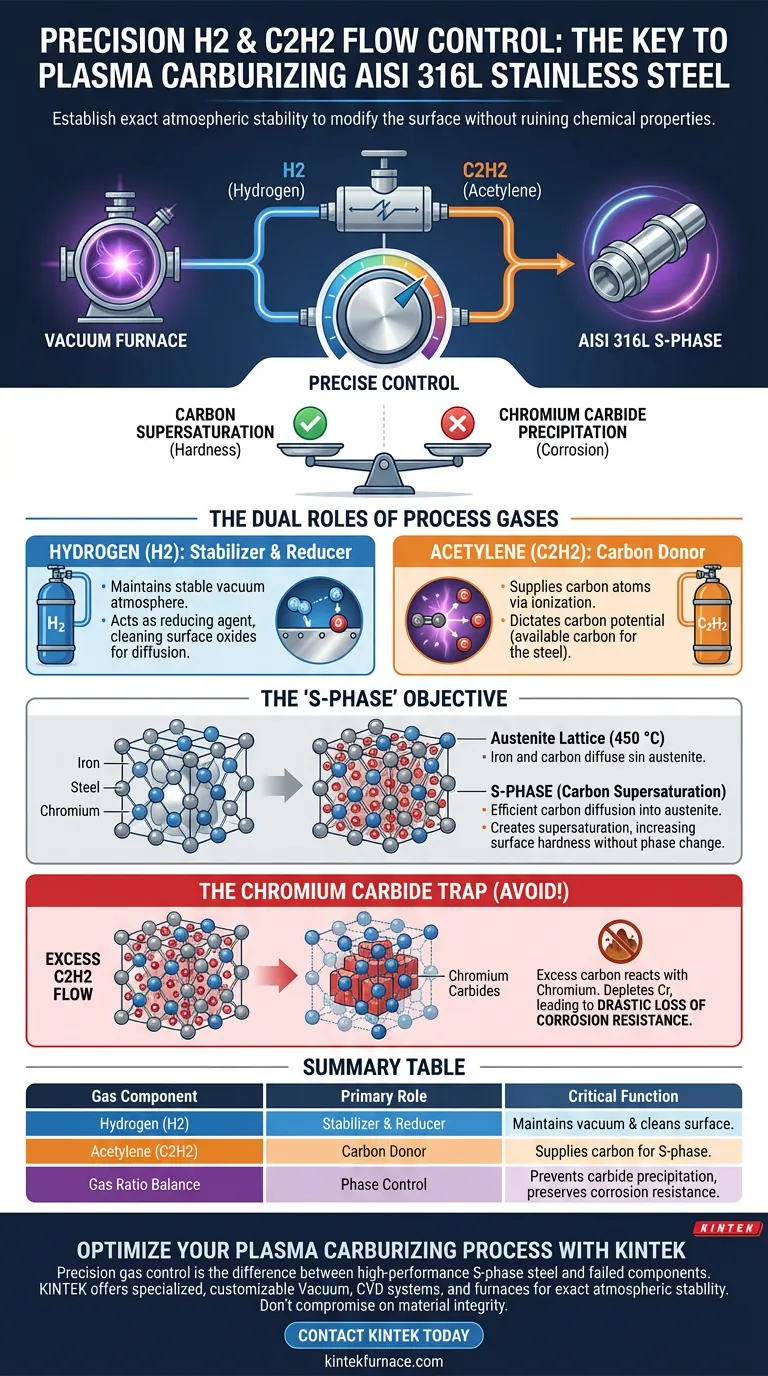
Precise control of Hydrogen (H2) and Acetylene (C2H2) flow rates is critical because it establishes the exact atmospheric stability required to modify the surface of AISI 316L stainless steel without ruining its chemical properties. This regulation ensures the correct concentration of carbon is available to diffuse into the steel's lattice structure while preventing the chemical reactions that lead to corrosion.
The success of plasma carburizing relies on a delicate equilibrium: generating enough carbon to achieve supersaturation for hardness, while strictly limiting carbon levels to prevent the precipitation of chromium carbides.
The Dual Roles of the Process Gases
To understand why flow rate precision is non-negotiable, you must first understand the distinct function of each gas within the furnace environment.
Hydrogen (H2): The Stabilizer and Reducer
Hydrogen acts as the carrier gas in this mixture. Its primary function is to maintain a stable atmosphere within the vacuum furnace.
Additionally, H2 serves as a reducing agent. It helps create the conditions necessary for clean diffusion by interacting with surface oxides, ensuring the steel surface is receptive to the carburizing process.
Acetylene (C2H2): The Carbon Donor
Acetylene serves as the carbon source. In the high-energy plasma environment, this gas is ionized to release carbon atoms.
These atoms are the active ingredients that diffuse into the surface of the AISI 316L specimen. The flow rate of C2H2 directly dictates the carbon potential of the atmosphere—essentially, how much carbon is available to enter the steel.
The "S-Phase" Objective
The ultimate goal of regulating these gases is to create a specific microstructural state known as the S-phase.
Achieving Carbon Supersaturation
When the flow rates are optimized, carbon atoms diffuse efficiently into the austenite lattice of the 316L steel.
Because the process occurs at a relatively low temperature (around 450 °C), these atoms become trapped in solid solution. This creates a state of carbon supersaturation, which significantly increases the surface hardness of the material.
Preserving the Lattice Structure
The S-phase is unique because it hardens the steel without changing its fundamental crystal structure.
To achieve this, the carbon concentration provided by the C2H2 flow must be high enough to fill the interstitial spaces in the lattice, but not so high that it forces a chemical phase change.
Understanding the Trade-offs: The Chromium Carbide Trap
The most critical reason for precise flow control is the avoidance of a specific, failure-inducing microstructural defect: chromium carbide precipitation.
The Consequence of Excess Carbon
If the C2H2 flow rate is too high, the concentration of carbon in the atmosphere exceeds the lattice's capacity to hold it in solution.
When this happens, the excess carbon reacts chemically with the chromium atoms present in the stainless steel.
The Loss of Corrosion Resistance
This reaction creates chromium carbides. While these are hard, their formation depletes the surrounding steel matrix of free chromium.
Since chromium is the element responsible for the "stainless" quality of the steel (by forming a passive oxide layer), its depletion leads to a drastic reduction in corrosion resistance. The steel may become hard, but it will essentially rust like plain iron.
Making the Right Choice for Your Goal
Achieving the S-phase requires navigating a narrow process window where temperature (450 °C) and gas ratios are strictly maintained.
- If your primary focus is Maximum Hardness: Prioritize the upper limit of the C2H2 flow rate to maximize carbon saturation, but verify the absence of carbide precipitates via microscopy.
- If your primary focus is Corrosion Resistance: Lean toward a conservative C2H2 to H2 ratio to ensure the austenite lattice remains free of chromium depletion, accepting slightly lower peak hardness.
Success in plasma carburizing is not about how much carbon you can add, but how precisely you can control its integration into the lattice.
Summary Table:
| Gas Component | Primary Role | Critical Function in Plasma Carburizing |
|---|---|---|
| Hydrogen (H2) | Stabilizer & Reducer | Maintains vacuum atmosphere and cleans surface oxides for diffusion. |
| Acetylene (C2H2) | Carbon Donor | Supplies carbon atoms for lattice supersaturation (S-phase development). |
| Gas Ratio Balance | Phase Control | Prevents chromium carbide precipitation to preserve corrosion resistance. |
Optimize Your Plasma Carburizing Process with KINTEK
Precision gas control is the difference between high-performance S-phase steel and failed, corroded components. At KINTEK, we understand the delicate equilibrium required for advanced material science.
Backed by expert R&D and manufacturing, KINTEK offers specialized Vacuum, CVD systems, and laboratory high-temperature furnaces—all fully customizable to provide the exact atmospheric stability and flow regulation your AISI 316L treatments demand. Don't compromise on material integrity.
Contact KINTEK today to discuss your custom furnace needs" Form)"
Visual Guide
References
- Lu Sun, Xiaomei Luo. Effect of Low-Temperature Plasma Carburization on Fretting Wear Behavior of AISI 316L Stainless Steel. DOI: 10.3390/coatings14020158
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Spark Plasma Sintering SPS Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Magnesium Extraction and Purification Condensing Tube Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What material treatments can be performed in a vacuum furnace? Achieve Clean, High-Quality Results
- How are vacuum furnaces applied in the semiconductor industry? Essential for High-Purity Chip Manufacturing
- What advantages does a vacuum drying oven offer over standard drying equipment for BHET recovery? Ensure Maximum Purity
- What are the key advantages of a vacuum environment in customized vacuum sintering furnaces? Achieve Purity, Strength, and Control
- How does a vacuum high-temperature furnace ensure structural stability? Unlock Precise Layered Porous Carbon Synthesis
- Why is vacuum brazing preferred in the aerospace industry? For Strong, Clean, and Reliable Joints
- How are vacuum furnaces environmentally friendly? Achieve Clean, Efficient Heat Treatment
- Why is a vacuum oven required for post-processing WTaVTiZrx alloy powders? Ensure Purity and Prevent Oxidation



















