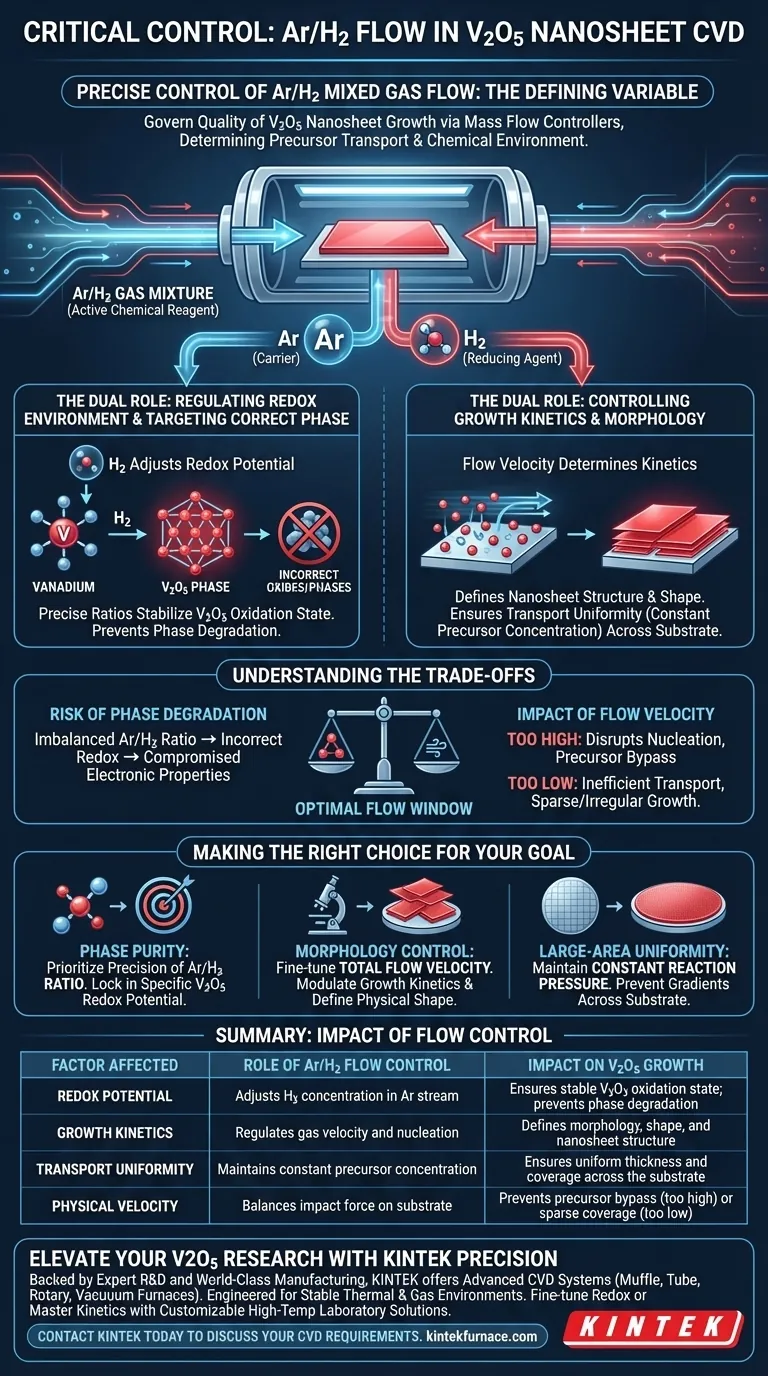
Precise control of Ar/H2 mixed gas flow is the defining variable that governs the quality of V2O5 nanosheet growth in Chemical Vapor Deposition (CVD). By strictly regulating the flow rates via mass flow controllers, you determine not only the physical transport of vanadium precursors but also the chemical environment required to stabilize the specific V2O5 oxidation state.
In this process, the gas mixture is not a passive carrier; it is an active chemical reagent. Mastering the flow rate allows you to simultaneously manage the kinetic delivery of vapor and the thermodynamic redox potential, ensuring the final material forms as uniform V2O5 nanosheets rather than unwanted phases.
The Dual Role of the Gas Mixture
To understand why precision is non-negotiable, you must view the Ar/H2 mixture as performing two distinct, simultaneous functions.
Regulating the Redox Environment
The introduction of Hydrogen (H2) into the Argon (Ar) stream acts as a reducing agent. This directly adjusts the redox potential within the reaction chamber.
Targeting the Correct Phase
This chemical adjustment is critical for guiding the VO2 vapor to the correct oxidation state. Precise ratios ensure the deposition settles specifically as V2O5, rather than over-reducing to other vanadium oxides or failing to react completely.
Controlling Growth Kinetics and Morphology
Beyond chemistry, the physical velocity of the gas flow dictates how the material constructs itself on the substrate.
Defining Nanosheet Structure
The flow rate determines the growth kinetics of the material. By controlling the velocity, you influence how atoms nucleate and arrange themselves, which directly determines the final morphology (shape and structure) of the nanosheets.
Ensuring Transport Uniformity
A stable flow acts as a carrier to transport vanadium vapor from the source to the substrate. Just as with other CVD processes (such as WS2 or MoS2 growth), consistent flow maintains constant precursor concentrations, ensuring the nanosheets are uniform in thickness across the entire sample.
Understanding the Trade-offs
Achieving the perfect growth condition requires balancing competing physical and chemical forces. Deviating from the optimal flow window creates specific risks.
The Risk of Phase Degradation
If the flow rate or ratio shifts, the redox potential changes. An imbalance here can lead to the deposition of incorrect vanadium phases, compromising the material's electronic properties.
The Impact of Flow Velocity on Deposition
If the flow velocity is too high, the physical impact force may disrupt the nucleation process or blow precursor vapor past the substrate. Conversely, if the flow is too low, the transport becomes inefficient, leading to sparse coverage or irregular growth patterns.
Making the Right Choice for Your Goal
When configuring your Mass Flow Controllers (MFCs) for V2O5 growth, align your settings with your specific experimental objectives.
- If your primary focus is Phase Purity: Prioritize the precision of the Ar/H2 ratio to lock in the specific redox potential required for V2O5 formation.
- If your primary focus is Morphology Control: Fine-tune the total flow velocity to modulate growth kinetics and define the physical shape of the nanosheets.
- If your primary focus is Large-Area Uniformity: Ensure your MFCs act to maintain constant reaction pressure and precursor concentration to prevent gradients across the substrate.
Success in V2O5 CVD relies on treating the gas flow as a tunable tool that bridges the gap between chemical potential and physical structure.
Summary Table:
| Factor Affected | Role of Ar/H2 Flow Control | Impact on V2O5 Growth |
|---|---|---|
| Redox Potential | Adjusts H2 concentration in Ar stream | Ensures stable V2O5 oxidation state; prevents phase degradation |
| Growth Kinetics | Regulates gas velocity and nucleation | Defines morphology, shape, and nanosheet structure |
| Transport Uniformity | Maintains constant precursor concentration | Ensures uniform thickness and coverage across the substrate |
| Physical Velocity | Balances impact force on substrate | Prevents precursor bypass (too high) or sparse coverage (too low) |
Elevate Your V2O5 Research with KINTEK Precision
Precise flow control is the difference between high-quality V2O5 nanosheets and failed experiments. Backed by expert R&D and world-class manufacturing, KINTEK offers advanced CVD systems—including customizable Muffle, Tube, Rotary, and Vacuum furnaces—engineered to provide the stable thermal and gas environments your research demands.
Whether you need to fine-tune redox potentials or master growth kinetics, our high-temp laboratory solutions are built to your unique specifications. Contact KINTEK today to discuss your CVD requirements and see how we can optimize your material synthesis.
Visual Guide
References
- Gangtae Jin. Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. DOI: 10.3390/nano15070548
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why must high vacuum and slow deposition rates be maintained for gold deposition? Unlock Precision Plasmonics
- Why is a solvent bubbler used in CVD for 2D COF synthesis? Optimize Polymerization & Crystallinity
- What is chemical vapor deposition (CVD) and how does it work? Discover High-Performance Film Growth for Your Lab
- What types of materials can be synthesized using the described CVD systems? Explore Versatile Synthesis for Advanced Materials
- What role does CVD play in the semiconductor industry? Essential for Building Advanced Microchips
- What biomedical applications do CVD furnaces have? Enhance Implant Safety and Drug Delivery
- What are some applications of CVD in various industries? Discover How CVD Transforms Materials for High-Tech Uses
- How does argon serve as a carrier gas during CVD of BN@PyC aerogels? Essential Tips for Uniform Deposition



















