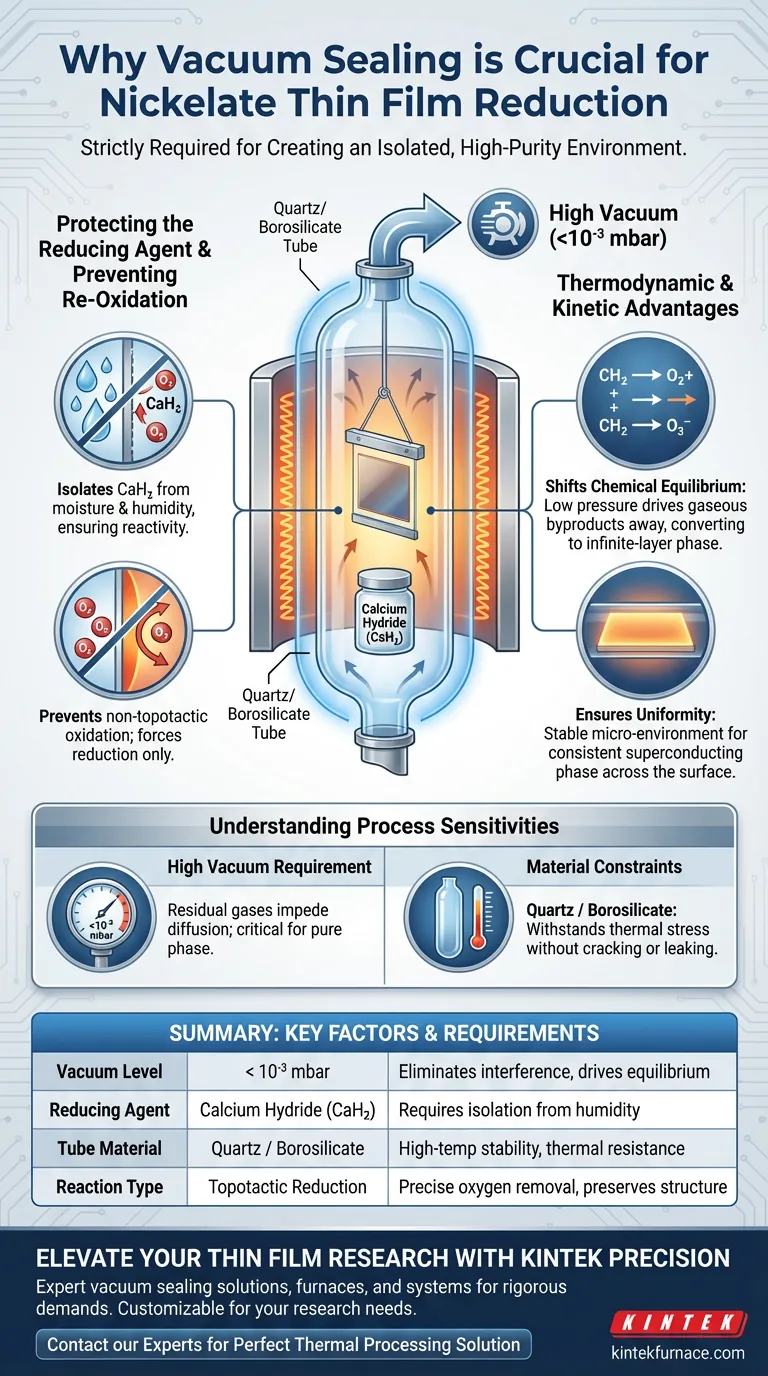
Vacuum sealing is strictly required to create the isolated, high-purity environment necessary for the successful topotactic reduction of nickelate thin films. By evacuating a glass tube to a high vacuum (typically below $10^{-3}$ mbar), you eliminate atmospheric moisture and oxygen that would otherwise degrade the reducing agent and destroy the sample. This sealed, negative-pressure environment is the only way to drive the chemical equilibrium toward the formation of the desired infinite-layer phase.
The vacuum seal acts as a critical barrier that preserves the chemical integrity of the calcium hydride reducing agent while creating the precise thermodynamic conditions required to strip oxygen from the lattice without collapsing the film structure.

The Critical Role of Environmental Isolation
Protecting the Reducing Agent
The reduction process relies heavily on Calcium Hydride (CaH2). This material is highly sensitive to moisture and will degrade rapidly if exposed to standard atmospheric conditions.
Vacuum sealing isolates the CaH2 within the glass tube. This prevents it from reacting with ambient humidity, ensuring it remains active enough to perform the reduction.
Preventing Re-Oxidation
At the high temperatures required for this reaction, nickelate films are prone to non-topotactic oxidation. This means the material could absorb oxygen from the air rather than losing it.
Sealing the tube prevents oxygen re-entry. This effectively forces the reaction to proceed in only one direction—reduction—rather than battling against atmospheric oxidation.
Thermodynamic and Kinetic Advantages
Shifting Chemical Equilibrium
The vacuum environment does more than just protect the materials; it actively drives the reaction. The low pressure facilitates the diffusion of gaseous reaction products away from the film.
By removing these gaseous byproducts, the system shifts the chemical equilibrium forward. This shift is essential for fully converting the material into the infinite-layer phase.
Ensuring Uniformity
According to supplementary data, the sealed quartz or borosilicate tube creates a stable micro-environment.
This encapsulation ensures that the reduction proceeds uniformly across the entire film surface. Uniformity is a prerequisite for obtaining a pure superconducting phase.
Understanding the Process Sensitivities
The Requirement for High Vacuum
Achieving a "rough" vacuum is often insufficient. The primary reference emphasizes a high vacuum of less than $10^{-3}$ mbar.
Failure to reach this pressure threshold leaves residual gas molecules in the tube. These residuals can impede the diffusion process or chemically alter the film surface.
Material Constraints
The process relies on specific glass types, such as quartz or borosilicate, to withstand the thermal stress of the furnace.
This adds a layer of complexity, as the sealing process itself must be robust enough to hold the vacuum throughout the high-temperature annealing cycle without cracking or leaking.
Making the Right Choice for Your Goal
To ensure the success of your nickelate reduction, consider the following based on your specific objectives:
- If your primary focus is achieving superconductivity: Prioritize a high-vacuum seal ($<10^{-3}$ mbar) to ensure the thorough removal of oxygen required for a pure phase.
- If your primary focus is process consistency: Use high-quality quartz or borosilicate tubes to create a repeatable, thermally stable micro-environment for every batch.
Strict adherence to vacuum sealing protocols is the difference between a degraded sample and a functional infinite-layer nickelate.
Summary Table:
| Factor | Requirement | Purpose |
|---|---|---|
| Vacuum Level | < 10⁻³ mbar | Eliminates residual gas interference and drives equilibrium. |
| Reducing Agent | Calcium Hydride (CaH₂) | Highly reactive agent requiring isolation from humidity. |
| Tube Material | Quartz / Borosilicate | High-temperature stability and thermal stress resistance. |
| Reaction Type | Topotactic Reduction | Precise oxygen removal without collapsing film structure. |
Elevate Your Thin Film Research with KINTEK Precision
Don't let atmospheric contamination compromise your superconducting materials. Backed by expert R&D and manufacturing, KINTEK offers specialized vacuum sealing solutions, Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of nickelate reduction. Our lab high-temp furnaces are fully customizable to meet your unique research needs.
Ready to achieve high-purity phase results? Contact our technical experts today to find the perfect thermal processing solution for your laboratory.
Visual Guide
References
- Araceli Gutiérrez‐Llorente, Lucía Iglesias. Toward Reliable Synthesis of Superconducting Infinite Layer Nickelate Thin Films by Topochemical Reduction. DOI: 10.1002/advs.202309092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What role do vacuum-sealed high-purity silica ampoules play in phase equilibrium experiments? Enhance Sample Integrity
- What is the primary function of an industrial vacuum drying oven in Si-RuO2 catalyst preparation? Achieve Uniformity.
- What functions do alumina crucibles and quartz tube encapsulation serve? Essential Shields for Na2In2As3 Synthesis
- What is the function of a high-precision constant temperature oven in LIG composite curing? Achieve Perfect Stability
- Why is the vacuum sealing of quartz tubes essential? Secure PdSe2 Growth and High-Temperature Safety
- What are the primary functions of a quartz tube reactor? Enhance Hydrogen Production and Induction Efficiency
- Why use a covered crucible for g-C3N4 calcination? Enhance Surface Area via Self-Exfoliation
- What are the advantages of using aluminum crucibles for siloxane research? Maximize Thermal Precision and Data Accuracy



















