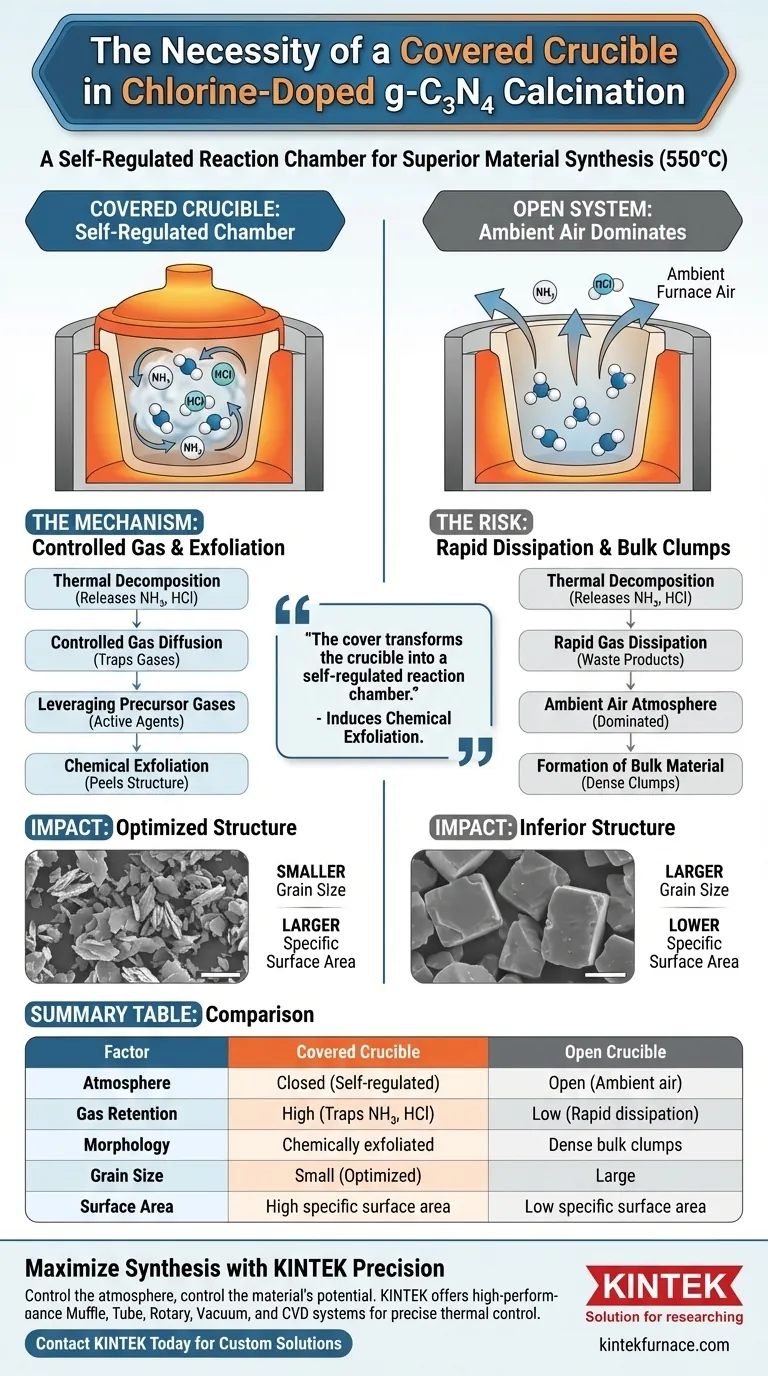
The use of a covered crucible is a necessity, not a preference, because it fundamentally alters the reaction atmosphere during the 550°C calcination process. By mechanically restricting the airflow, you prevent the rapid escape of decomposition gases, forcing them to actively participate in refining the material's structure.
The cover transforms the crucible into a self-regulated reaction chamber. By modulating the diffusion of ammonia and hydrogen chloride, you induce a chemical exfoliation process that is impossible to achieve in an open system.

The Mechanics of a Closed Reaction Atmosphere
Controlling Gas Diffusion
When calcining precursors for chlorine-doped graphitic carbon nitride, the material undergoes thermal decomposition. This releases volatile gases.
A covered crucible creates a relatively closed environment. This setup significantly slows the diffusion speed of these gases, preventing them from immediately dissipating into the wider furnace chamber.
Leveraging Precursor Gases
The specific gases generated during this decomposition include ammonia ($NH_3$) and hydrogen chloride ($HCl$).
In an open crucible, these gases would be waste products. In a covered crucible, they become active agents. The lid traps these gases at high concentrations directly around the reacting solid.
Impact on Material Structure
Facilitating Self-Exfoliation
The retention of high-temperature gases creates a unique chemical environment. The trapped $NH_3$ and $HCl$ interact with the bulk material.
This interaction causes the gases to exfoliate the bulk structure. Instead of forming large, dense clumps, the material is chemically peeled apart by its own decomposition byproducts.
Optimizing Grain Size and Surface Area
The physical result of this gas-assisted exfoliation is a dramatic change in morphology.
The process yields smaller grain sizes compared to open-air calcination. Consequently, this reduction in grain size leads to a larger specific surface area, which is a critical metric for the catalytic performance of graphitic carbon nitride.
Understanding the Trade-offs
The Risk of Open Systems
It is important to understand what happens if the cover is omitted. Without the lid, the reaction atmosphere is dominated by the ambient furnace air rather than the precursor gases.
The diffusion of $NH_3$ and $HCl$ becomes too rapid to effect change. The result is a "bulk" material with larger grains, lower surface area, and likely inferior electronic or catalytic properties.
Consistency vs. Pressure
While the cover is necessary, it creates a variable pressure environment.
You must ensure the crucible material can withstand the specific chemical attack of hot $HCl$ gas. However, for standard synthesis of this material, the benefits of the "self-exfoliation" mechanism far outweigh the equipment demands.
Making the Right Choice for Your Goal
When setting up your high-temperature furnace, consider your specific material requirements:
- If your primary focus is high catalytic activity: Always use a covered crucible to maximize specific surface area through gas-assisted exfoliation.
- If your primary focus is studying bulk properties: You might opt for an open crucible to minimize exfoliation, though this will result in a material with larger grain sizes.
Control the atmosphere, and you control the material's potential.
Summary Table:
| Factor | Covered Crucible | Open Crucible |
|---|---|---|
| Atmosphere | Closed (Self-regulated) | Open (Ambient air) |
| Gas Retention | High (Traps $NH_3$, $HCl$) | Low (Rapid dissipation) |
| Morphology | Chemically exfoliated | Dense bulk clumps |
| Grain Size | Small (Optimized) | Large |
| Surface Area | High specific surface area | Low specific surface area |
Maximize Your Material Synthesis with KINTEK Precision
Achieving the perfect chemical exfoliation requires precise thermal control. At KINTEK, we understand the nuance of atmosphere-controlled reactions. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to support your specific chlorine-doped graphitic carbon nitride research.
Ready to elevate your lab's catalytic research? Contact us today to discover how our high-temperature furnaces can provide the stability and precision your unique projects demand.
Visual Guide
References
- Jie Ji, Ren Qian Tee. Chlorine-Doped Graphitic Carbon Nitride for Enhanced Photocatalytic Degradation of Reactive Black 5: Mechanistic and DFT Insights into Water Remediation. DOI: 10.1021/acsomega.5c04017
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What key roles do high-purity graphite molds play in SPS? Powering High-Entropy Carbide Synthesis
- Why are laboratory hydraulic presses critical for FMDS pelletization? Boost Strength Without Heat
- How does heating equipment with magnetic stirring contribute to Fe3O4 synthesis? Achieve Precise Nanoparticle Control
- What materials are commonly used for furnace tubes to withstand high heat? Choose the Best for Your Lab
- Why is a vacuum pump utilized in research concerning the reaction of magnesium with carbon dioxide and nitrogen? Ensure Data Integrity
- How does the selection of a ceramic crucible contribute to the preparation of biomass carbon catalysts? Maximize Purity
- What is the function of a rotary evaporator in the recovery of formic acid lignin? Preserve Quality & Boost Efficiency
- What is the function of a laboratory electric blast drying oven in biomass pretreatment? Standardize Your Samples



















